Immunotherapies have shifted the paradigm for cancer treatment through their ability to induce long-term remission in blood cancer patients for whom other treatments have failed. Thanks to novel treatments such as CAR-T therapies, these patients are living longer after a single infusion, with a better quality of life—something that would have previously been unthinkable.
“People are so excited about it as it really provides hope that cancer can one day be cured,” says Frank Zhang, chair of Legend Biotech, which has successfully treated multiple myeloma patients with a CAR-T therapy. “But now we want to reach more patients.”
As demand for CAR-T therapy grows and regulatory agencies approve broader use and in earlier lines of treatment, manufacturers will need to devise ways to produce more CAR-T therapies faster. Because current CAR-T therapies are personalized treatments derived from a patient’s own cells, creating them is a time-consuming and heavily regulated process. Zhang and others in the field are seeking ways to expedite getting CAR-T to patients.
Striking cancer early
Immunotherapies, like all cancer therapies, are more likely to be effective early in the disease, before a cancer has spread too far or grown too much, and while the cancer is more likely to lose a battle with the immune system. But that creates a dilemma. As experimental therapies move through clinical trials, they are tried first on sicker patients—those whose cancer has recurred following treatment with more established therapies, and is therefore harder to overcome.
This dilemma can be overcome in part through clinical trials, which can give regulators confidence that the new therapy is more effective than an established therapy. For example, in April, following a phase 3 clinical trial, the FDA approved Legend’s CAR-T therapy for multiple myeloma, ciltacabtagene autoleucel (Carvykti), to treat patients whose cancer had returned following initial rounds of treatment.
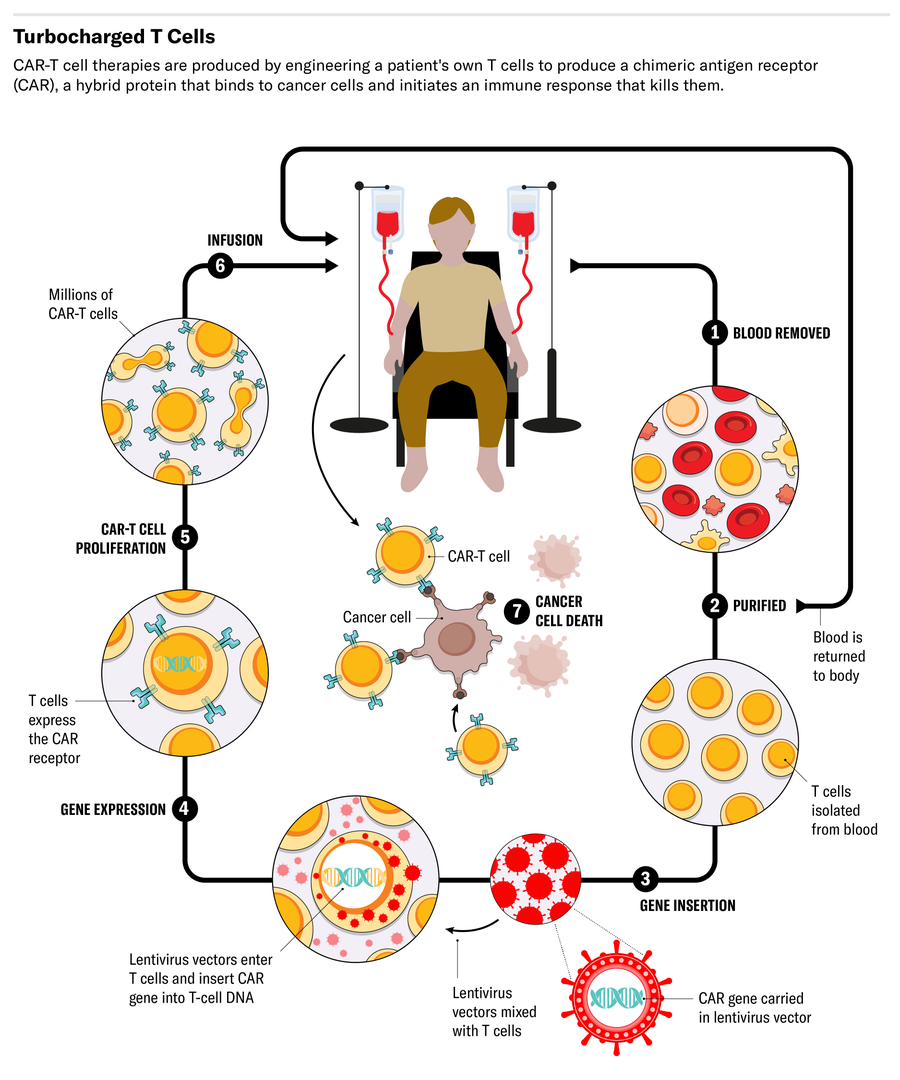
Current CAR-T therapies, known as autologous therapies, harness the power of a cancer patient’s own immune system. To produce them, immune cells called T cells must first be collected from the patient and cultured in a manufacturing facility. These white blood cells form the basis of the body’s adaptive immune response to fight disease-causing pathogens.
Once isolated, these T cells must be engineered to produce proteins on their surface called chimeric antigen receptors, or CARs. These CARs recognize and bind to specific proteins (antigens) on the surface of cancer cells, which allows the modified T cells to bind and destroy them.
The engineered CAR-T cells are then grown until technicians can harvest enough to be therapeutic. Finally, they must be infused back into the patient, where they act as a living drug that can turbocharge the immune system’s ability to clear tumor cells.
The whole process—from collecting the immune cells to infusing the CAR-T cells back into the patient—takes weeks. That delay limits the throughput of cell manufacturing facilities, placing a cap on the number of patients they can treat. And it’s too long for some severely ill patients. “Because their cancer progresses very fast, many patients cannot wait,” Zhang says.
Scaling production
One way to deliver CAR-T therapy to more patients is to increase capacity by reducing the time needed to process each batch of cells. Each step of the production chain has scope for optimization, says Ying Huang, CEO of Legend.
“We’re attacking that problem from quite a few different angles,” Huang says. One is automation. The company is planning to test robotic arms to replace repetitive manual steps such as pipetting to help engineer the cells. “A robot can do that 24-7. They don't get tired from standing there,” he says.
Another advance could come from something as simple as a larger bioreactor to grow the viruses used to help engineer the patient’s T cells. Called lentiviruses, these are needed to transduce the therapeutic chimeric antigen receptor into the T cells.
Lentiviruses for CAR-T cell development are traditionally grown in one- or five-litre flasks. That may offer sufficient numbers for a CAR-T trial of a hundred people or so. “But now we’re trying to serve a few thousand patients a year,” Huang says. Legend and Johnson & Johnson have collaborated in developing a 50-liter bioreactor that can produce lentiviruses from cells grown in a liquid suspension. “That means we won’t have to worry about shortages or constraints on supply of lentivirus.”
Off-the-shelf CAR-T cells
There are dozens of other potential ways to enable CAR-T cell therapies to treat more people, says Bruce Levine, who works on novel cell therapies at the University of Pennsylvania.
One is to analyze the quality of a patient’s T cells as a way to help predict which will better respond to the treatment. “The higher percentage of T cells that have those characteristics, the higher the quality of the product and the more likely that we'll see a response,” he says.
Another is to find ways to engineer T cells that do not require lentivirus. His lab, for example, is using lipid nanoparticles to transfer the genetic material that boosts the performance of a patient’s T cells. “You get very specific uptake of the lipid nanoparticle that’s targeted to T cells, in the absence of targeting other cells,” he says.
Such off-the-shelf, or allogeneic, CAR-T cells could treat more patients than today’s CAR-T cells because they would be manufactured in advance, stored and then used when necessary—in a similar way to most other drugs.
But there is a major hurdle in this approach, Zhang says. A patient’s immune system can recognize the cells as foreign and attack them, so allogeneic therapies need to be engineered to avoid detection and be both effective and safe.
Zhang says it will take more time and research to perfect the allogeneic technique, “Right now, if we use a patient's own cells, the efficacy is so good. To compete, any next drug will have to somehow match that performance.”
Alisdair Macdonald
Learn more about CAR-T technologies at this dedicated site from Legend Biotech.