Abstract
We report on the fabrication and characterization of a highly sensitive pressure sensor using a Au film patterned on a polydimethylsiloxane (PDMS) membrane. The strain-induced change in the film resistance was utilized to perform the quantitative measurement of absolute pressure. The highest sensitivity obtained for a 200 µm thick PDMS film sensor was 0.23/KPa with a range of 50 mm Hg, which is the best result reported so far, over that range, for any pressure sensor on a flexible membrane. The noise-limited pressure resolution was found to be 0.9 Pa (0.007 mm Hg), and a response time of ∼200 ms, are the best reported results for these sensors. The ultrahigh sensitivity is attributed to the strain-induced formation of microcracks, the effect of which on the resistance change was found to be highly reversible within a certain pressure range. A physical model correlating the sensitivity with the sensor parameters and crack geometry has been proposed.
Export citation and abstract BibTeX RIS
1. Introduction
Highly sensitive thin film pressure sensors utilizing either rigid or compliant substrates have wide applications in several different fields including bio-implantable devices. Past research efforts have been mostly directed toward developing pressure sensors based on either conventional silicon or biocompatible materials [1–12]. Typically, thin film pressure sensors are based on changes in either resistance [1–3, 13] or capacitance [5–11]. Resistive pressure sensors detect the change in resistance caused by the pressure-induced deformation of the thin film resistor. Lim et al [1] designed and fabricated a flexible membrane pressure sensor with a patterned piezoresistive amorphous silicon (a-Si:H) thin film. Lee and Choi [2] demonstrated a polydimethylsiloxane (PDMS) diaphragm-based pressure sensor using a carbon fiber. Integration of graphene films on PDMS has also been performed to develop stretchable and biocompatible strain gauges [3]. The gauge factor of the strain gauge was 6.1, which is better than that of conventional strain gauges based on metal alloys of ∼2 [4]. Although pressure sensors based on a piezoresistive effect have a simple structure and long-term stability, their limited measurement range, and difficulty in integrating the piezoresistive material and flexible biocompatible membrane are major challenges that limit their further applications.
Capacitive pressure sensors have been intensely investigated by several research groups using both silicon [5–7] and flexible membranes [8–11]. Leineweber et al [5] developed a tactile sensor chip to measure the distribution of forces that act on its surface. The transducer element is a dynamic capacitor, which consists of a polysilicon membrane as a floating electrode and a bottom electrode implanted in the silicon substrate. Simons et al [6] also reported an implantable biosensor based on a flexible silicon dielectric membrane, which monitors pressure changes by sensing the capacitance changes between two counter electrodes. Zhou et al [7] designed and fabricated a highly sensitive capacitor pressure sensor with a sandwich structure, drastically reducing the processing complexity associated with fabricating these sensors. Although silicon-based thin film pressure sensors are popular in the field of sensor development, pressure sensors and strain gauges based on compliant biocompatible materials, such as PDMS, parylene, polyethylene terephthalate, polymide, etc, are also being extensively investigated in recent years due to biosensing applications [1–3, 8–11].
Of the biocompatible materials, PDMS has emerged as the material of choice for biosensor development due to its high flexibility [2, 14], well-known biocompatibility [12, 15], chemical inertness [16, 17] and ease of molding [12, 18]. It offers much higher sensitivity, especially in a low-pressure region, due to lower Young's modulus, which has prompted its usage in a variety of bio-implantable pressure sensors [6, 19, 20] and artificial skin [21–25] applications. Cheng et al [8] presented a capacitive tactile sensor with a floating counter electrode on the PDMS thin membrane with top sensitivity of 0.02/KPa within 420 mm Hg. The most sensitive pressure sensor so far, considering all types of pressure sensors, has been reported recently by Mannsfeld et al [9], where they obtained a maximum sensitivity of 0.55/KPa under 15 mm Hg. They used a capacitive detection method utilizing three-dimensional micromachined PDMS microstructures. Although these pressure sensors demonstrated excellent performance in the low-pressure regime (<75 mm Hg) [10], their response was highly nonlinear, with much reduced sensitivity (0.15/KPa) at higher pressure. Their fabrication also involved complicated processing steps, and being three-dimensional, they are not suitable for the integration in small implantable sensors.
The sensors reported so far suffer from one or more limitations of high fabrication complexity, low sensitivity, small measurement range, complicated structure and difficulty in miniaturization. Thus, there is a strong need for developing a low-cost and fully biocompatible pressure sensor which exhibits very high sensitivity in the low-pressure region (<10 KPa). In this paper, a highly sensitive planar pressure sensor based on resistance changes of Au film resistors, patterned on flexible PDMS membranes, has been fabricated and investigated. We obtained a constant sensitivity of 0.23/KPa over a range of 0–50 mm Hg, which yielded a noise-limited resolution of 0.9 Pa (0.007 mm Hg) and a response time of ∼200 ms. To the best of our knowledge, the sensor characteristics reported here are the best so far for biosensing applications. The sensor has been extensively modeled through COMSOL-based finite element simulations, for the first time, for design and performance prediction. The exceptional sensor characteristics including extremely high sensitivity, pressure resolution and very low response time, coupled with simple construction, ease of fabrication and biocompatibility, make them highly promising for a wide range of biomedical applications. The small footprint (3 × 3 mm2) also allows for high pixel density over large area for artificial skin applications.
2. Experimental methodology
The PDMS thin film-based pressure sensor was developed following a systematic approach that involved extensive COMSOL-based finite element simulation, followed by device fabrication and characterization. These are discussed in detail in the following sections.
2.1. Sensor design and modeling
To enable bio-implantable applications of these sensors, their effective sensing area was kept at 3 × 3 mm2, with the thickness of the PDMS thin film as 200 µm. The total sensor size was kept at 10 mm × 10 mm × 5 mm (length × width × thickness) for ease of handling, which can be easily reduced further. The schematic diagram of the sensor is shown in figure 1. The PDMS frame walls and the thin membrane enclose an air chamber with dimensions 3 × 3 × 2 mm3, which was used to put desired pressure on the PDMS thin membrane. A polyvinyl chloride (PVC) tube with an outer diameter of 3/32 inch was attached to the sidewall of the cavity through a hole on the PDMS wall (figure 1). A serpentine Au thin film resistor was patterned on the top surface of the PDMS membrane using a laser-patterned shadow mask. A serpentine geometry was chosen to maximize the length of the Au film resistor (which would also enhance sensitivity), while minimizing the sensor footprint. The width, length and thickness of the Au film stripe were 100 µm, 17.5 mm and 200 nm, respectively. Two 1 mm × 1 mm contact pads were deposited at the two ends of the resistor to establish external contacts.
Figure 1. (a) Three-dimensional schematic diagram of the PDMS thin membrane-based pressure sensor. (b) The top view of the sensor with the dimensions.
Download figure:
Standard imageAn important parameter for the sensor design is the thickness of the PDMS membrane. A thinner membrane can offer higher sensitivity, but due to larger deformation, will have a limited pressure range of operation, beyond which irreversible damage to the Au film can occur. The maximum strain, without causing the Au film to crack, has been reported by Wen and co-workers [13, 26] to be 0.8%. Based on their results, the maximum strain for our sensor was selected as 0.8% for a reliable operation. For biosensing applications, we chose the pressure range of operation for these sensors to be 0–50 mm of Hg (6.67 KPa). COMSOL-based finite element simulations were performed to determine the maximum strain at the Au film stripe. To characterize the PDMS deformation, we used a nonlinear Moony–Rivlin stress–strain relationship, instead of a traditional linearly elastic stress–strain function, since the Moony–Rivlin relationship is uniquely suited for rubber-elastic deformations [27, 28]. As shown in figure 2(a), when the pressure was applied, the PDMS membrane was deformed in three dimensions, and thus the induced strain distribution is not uniform on the Au resistor pattern. Generally, strain at the central area is larger than that at the edges, but the maximum strain occurs at the junctions between the serpentine resistor and the pads. Figures 2(b) and (c) show the strain distribution along the X–X' and Y–Y' lines (marked in figure 1(a)) on the top surface of the Au resistor. Figure 2(d) shows the variation of the maximum strain with the thickness of the PDMS membrane at an applied pressure of 50 mm Hg. We find that for the PDMS thickness less than 175 µm, the maximum strain is larger than 0.8% at 50 mm Hg. Therefore, for a full range of operation up to 50 mm Hg, the PDMS film thickness was chosen to be 200 µm in this work.
Figure 2. (a) Strain distribution on the top surface of the Au resistor on the PDMS membrane of the sensor. (b) Strain distribution on the Au resistor along the X–X' line. (c) Strain distribution on the Au resistor along the Y--Y' line. (d) Variation of the maximum strain with the thickness of the PDMS membrane.
Download figure:
Standard image2.2. Sensor fabrication
The pressure sensor was realized by bonding a PDMS thin membrane to an air chamber made of PDMS thick walls. The detailed fabrication steps for the PDMS-based pressure sensor are shown in figure 3(a). PDMS preparation was done by mixing the elastomer base and curing agent in the weight ratio 10:1 using the Sylgard 184 silicone elastomer kit (Dow Corning Co.). A 200 µm thick PDMS membrane was spin-coated on an Si wafer with a layer of photoresist (PR) 1811 and then baked at 95 °C for 5 h. Acetone was applied to solve the sacrificial PR layer when the PDMS thin membrane was being peeled off (step 1). The chamber with thick PDMS walls was built using a molding method in an aluminum mold (step 2). A PVC tube (Tygon®) was mounted on the wall for air inlet and outlet. The PDMS thin membrane was then bonded onto the chamber (step 3). The Au pattern was deposited using electron beam evaporation (DV-502A, Denton Co.) and a laser-cut polyimide film (Kapton®, DuPont Co.) as the shadow mask with 300 µm in thickness (steps 4 and 5). Before Au film deposition, no additional surface preconditioning was performed on the PDMS membrane. Since the PDMS film copied the low surface roughness (SR) of the silicon substrate (1 nm in our case), its SR would at most be a few nanometers, which is not expected to impact the mechanical properties or roughness of the 200 nm thick gold layer and influence the microcrack formation. Figures 3(b) and (c) shows a well-defined Au serpentine resistor on a 200 µm thick PDMS thin membrane of a pressure sensor.
Figure 3. (a) Fabrication process flow diagram of the pressure sensor. (b) Optical image of the pressure sensor with a 200 nm thick Au serpentine pattern. (c) Magnified image of the Au pattern.
Download figure:
Standard image2.3. Sensor characterization
The experimental setup for the sensor characterization is shown in figure 4. The pressure sensing tests were conducted using a Cascade Microtech probe station using the Agilent data acquisition system (Agilent 34972A). The pressure applied to the device was adjusted using a manual pressure pump (3D instruments, LLC), which was connected to the device using a PVC tube. A commercial pressure gauge (Omega, DPG5600B-05G) was mounted on the pressure pump to monitor the real air pressure in the device. A steady dc voltage of 0.5 V was supplied by Agilent E3649A to measure the resistance changes, and the data were acquired by a computer for processing.
Figure 4. Schematic diagram of the setup for sensor characterization.
Download figure:
Standard image3. Results and discussion
3.1. Sensitivity, gauge factor and resolution
The sensitivity S of the sensor is calculated as S = (ΔR/R0)/ΔP, where ΔR is the relative change in resistance, R0 is the original resistance and ΔP is the change in applied pressure on the thin PDMS membrane. The relative change in resistance with applied pressure for a typical sensor with a nominal thickness of 200 µm is shown in figure 5. We find that the response of the sensor is quite linear, and the sensitivity ((ΔR/R0)/ΔP = slope of the curve at a given pressure) remains almost constant at a value of 0.23/KPa (0.03/mm of Hg) over the entire pressure range of interest (up to 50 mm Hg). This is an extremely high sensitivity, and to our knowledge the highest so far reported on the PDMS-based resistive sensor. It is a few orders of magnitude higher compared to previously published results, which varied in the range 8 × 10−4−6 × 10−2/KPa [2, 7, 8, 11, 23, 29] for similar dimensions of sensing area (i.e. 0.6 × 0.6 mm2 −5 × 5 mm2) (table 1). Considering both resistive and capacitive sensors, this result is next only to the best result reported so far based on capacitive sensors by Mannsfeld et al [9] who reported a sensitivity of 0.55/KPa in the 0–15 mm Hg pressure range. Our sensitivity value is however better over the entire range of 0–50 mm of Hg studied, and is constant over that range, unlike that in [9], where it reduces very significantly to 0.15/KPa for higher pressure. For sensors with a thinner PDMS membrane (i.e. 100 µm thick), we expect the sensitivity to be even higher because the same pressure will cause larger deformation on the thinner PDMS membrane.
Figure 5. Relative resistance change with the applied pressure for the sensor, exhibiting very high sensitivity of 0.23/KPa. The inset shows the actual resistance changes with pressure.
Download figure:
Standard imageTable 1. Major sensor parameters in this work compared to those reported in recent years.
| References | Sensitivity (/KPa) | Size (mm2) | Thickness of membrane (µm) | Measurement range (KPa) | Linear range (KPa) |
|---|---|---|---|---|---|
| This work | 0.23 | 3 × 3 | 200 | 0–6.7 | 0–6.7 |
| [2] | 0.008 | 1 × 1 | 50 | 0–25 | 0–25 |
| [7] | 0.00084 | 1.5 × 1.5 | 5 | 80–106 | 80–106 |
| [8] | 0.0056 | 3 × 3 | 1000 | 0–720 | 0–240 |
| [11] | 0.058 | 4 × 1 | 5 | 0–4 | 0–4 |
| [23] | 0.005 | 0.6 × 0.6 | 470 | 0–16.7 | 0–5.6 |
| [29] | 0.02 | 5 × 5 | 50 | 0–200 | 0–200 |
| [9] | 0.55 | 8 × 8 | <100 | 0–7 | 0–2 |
To compare the performance of these sensors with other pressure sensors, it is necessary to calculate their strain gauge factor G. However, this calculation is not very straightforward as the PDMS-based flexible pressure sensor has a non-uniform strain distribution (figures 2(a)–(c)). Hence, the average strain across the metal thin film resistor, which was computed by COMSOL-based finite element simulation, was used to calculate the strain gauge factor as G = (ΔR/R)/εav. The average strain is defined as the arithmetic average of the maximum principal strains over the entire length of the resistor. Figure 6 shows the fractional change in resistance (ΔR/R) with the average strain. The slope of the plot in figure 6 gives the strain gauge factor G, which is determined as 1058. This is much higher than those of the commercial strain gauges based on thin metal wire (∼2), as well as those of the strain sensors reported earlier: 1.4–74, based on carbon nanotube/PDMS composites [30] or a deposited metal film on the PDMS membrane [13, 26]. A theoretical model explaining the ultrahigh sensitivity and gauge factor has been proposed in section 3.3.
Figure 6. Relative resistance change versus average strain on the Au thin film resistor. Slope of the curves yields the gauge factor of the pressure sensor G = 1058.
Download figure:
Standard imageTo determine the pressure measurement resolution of the sensor, the overall sensor resistance noise was measured. Figure 7 shows the resistance root mean square (RMS) noise at different applied pressures which can be seen to remain fairly constant with pressure. The RMS noise is calculated as 0.088 Ω at 0 mm Hg, making the noise-limited resolution of the sensor as 0.007 mm of Hg (or 0.9 Pa). This resolution is much better than what is necessary for blood pressure measurement, which typically requires a resolution of 0.3 mm Hg [31]. Moreover, the resolution of our sensor is higher than those of the pressure sensing elements reported earlier for artificial skin applications (3–3000 Pa) [8, 9, 23, 25].
Figure 7. The RMS noise level of the sensor at different pressures. The inset shows the resistance fluctuation plotted as a function of time, which was used in the RMS noise calculation.
Download figure:
Standard image3.2. Stability, response time and reliability
A faster response time of the pressure sensor is essential to obtain accurate real-time information about pressure. Figure 8 shows the response and relaxation times (rise and fall times, respectively) of the sensor corresponding to the loading and unloading (pressures on and off, respectively). In these measurements, the pressure was quickly adjusted manually from 0 to 5 mm Hg. The pressure was kept at 5 mm Hg for 30 s and then reduced back to 0 for 30 s, and the process was repeated for 300 times. Figure 8 shows the resistance change of the sensor in the 15 loading–unloading cycles. Clearly, for this pressure range, the sensor performance is very consistent over the various cycles. A single loading–unloading cycle is shown in figure 9, from which the loading and unloading response times (time gap between 10% and 90% of the steady state values at each pressure) for the sensor can be determined to be 175 and 205 ms, respectively. The unloading response time was found to be always slightly higher than the loading time, possibly due to the specific elastic behavior of the PDMS, and is consistent with earlier observations [9, 25, 32]. It should be noted that the response and relaxation times include the response times for the human hand, and thus represent upper limits for the sensor response times. Nonetheless, these response times are —one to three orders of magnitude better than previous sensors [33–35] and even better than the capacitive sensor results reported by Mannsfeld et al [9].
Figure 8. Sensor response of 15 consecutive loading and unloading cycles (60 s duration) between 0 and 5 mm Hg pressure.
Download figure:
Standard imageFigure 9. Sensor response of a single loading and unloading cycle (10 s duration) between 0 and 5 mm Hg pressures. The response times for loading and unloading are found to be 175 and 205 ms, respectively.
Download figure:
Standard imageTo determine the reliability of the sensor over time, we compared the sensor sensitivity characteristics over a period of three weeks, during which hundreds of loading and unloading processes were performed on the sensor for various pressure ranges. Figure 10 shows the relative resistance change of the same sensor in the beginning of our test and at the end of the three-week period. We find that the sensor responses are quite similar, although the response of the aged sensor after three weeks is more nonlinear compared to that at the beginning. We attribute this partially to the fatigue in the Au films, but mostly to the change in elastic behavior of the PDMS thin film. As mentioned before, the PDMS has a nonlinear stress–strain relationship [27, 28], which may become more prominent due to extensive stressing of the sensor. However, the nonlinearity is similar to that observed widely in capacitive sensors [5, 7–9, 23], and should not pose a significant problem for device operation; it can be easily calibrated.
Figure 10. Comparison of the sensor characteristics before and after extensive characterization involving hundreds of cycles.
Download figure:
Standard image3.3. Modeling of the sensor sensitivity
In general, the gauge factor is a result of both geometric deformation and change in electrical resistivity caused by strain. When a typical metal film resistor is subjected to strain, both the strain-induced changes of geometry and electrical resistivity are responsible for its resistance change. From the equation, R = ρL/A, where R is the resistance, ρ is the resistivity, L is the length and A is the area of the cross section, when the resistor is subjected to symmetric biaxial tensions, the gauge factor G of the strain sensor is given as
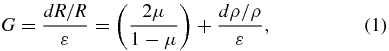
where ε is the axial strain, µ is Poisson's ratio of the material and dρ is the change of the resistivity of the material. If we neglect the change of resistivity, we find G ≤ 2 as µ ≤ 0.5 for all materials. For this reason, commercially available strain gauges have G ≈ 2, as the resistivity of the material does not change. Obtaining a gauge factor more than 2 therefore involves a large change in metal film resistivity. However, there is no accurate model describing the resistivity change of a metal due to pure geometric deformation. Wen and co-workers made a strain gauge sensor using a PDMS thin membrane with a deposited Au metal film resistor to measure surface strain on a living bone [13, 26]. These sensor devices, operating under uniaxial strain, had a gauge factor between 37 and 74, which is much larger than G = 2, given by equation (2) [13, 26].
Several reports in recent years have suggested that the formation of microcracks is the major reason for causing strain-induced resistivity changes in metal thin films on polymer or elastomeric materials [36–43]. When subjected to strain, the metal film cracks, but does not get completely disconnected, i.e. a very thin (sub-nanometer level, as will be discussed later) layer of metal at the bottom of the crack still conducts [36, 39]. However, this can give rise to a dramatic change in the film conductivity. The mechanism of formation of microcracks has not been understood clearly yet. The cracks possibly arise from the imperfections in the grain boundaries of the deposited metal film [41, 42]. Interestingly, experimental studies focusing on the stretchability of gold thin film on the PDMS substrate have revealed that the strain-induced cracks are reversible, up to external strains of tens of per cent, due to elastic recovery of the elastomeric substrate [37, 38]. Therefore, for our pressure sensor, which operates in the low-pressure range, e.g. 0–50 mm Hg (i.e. strain 0–0.8%), we can expect the microcrack formation to be quite reversible. In our experiments, we observed that some initial microcracks are formed spontaneously following the deposition of the Au film, and more created in the Au layer when the PDMS membrane was subjected to pressure. The microcracks mostly grow perpendicular to the Au stripes as seen from figures 11(b) and (c), which also makes the width of the cracks approximately equal to the width of the Au stripes.
Figure 11. (a) Three-dimensional schematic diagram of a strain-induced microcrack in the metal film. (b) An SEM image of the Au resistor on a representative pressure sensor at 24 mm Hg pressure. The inset shows a magnified section of the Au resistor, clearly showing several microcracks. (c) Magnified view of a single microcrack on one of the Au stripes. The inset shows the magnified image of the crack. The length and width of the crack are 0.8 and 104 µm, respectively.
Download figure:
Standard imageBased on the formalism developed in [39], we have developed a simple model to estimate the strain gauge G of a thin metal film resistor on an elastomeric substrate. As shown in figure 11(a), because the thickness of the residual film Tc at the bottom is far less than that of the original metal film T0, the resistance change due to the pure geometric change (i.e. first term in equation (1)) is very small compared to the increase in resistance across the cracks, and hence neglected. Assuming Tc to be constant, the resistance change ΔR at a certain strain ε is the sum of resistance change caused by all the cracks, and given as

where ρ is the resistivity, and Lci and Wci are the length and width of the ith crack, respectively (see figure 11). Since the sum of all the microcrack lengths should be equal to the change in the length of the metal film under strain ΔL, we have ∑Lci = ΔL = εL0. Substituting this relationship in equation (2), and putting Wci to be equal to W0 (see the earlier discussion), we find

where W0 and L0 are the original width and length of the medal resistor line. Assuming that the resistivity of the material in the crack is the same as the bulk material, the gauge factor G simply becomes
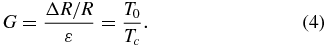
Equation (4) gives a very simple expression of the gauge factor of a metal thin film on an elastomeric substrate involving only the thicknesses of the metal film resistor and the residual film thickness in the microcracks. The thickness of the metal film resistor used in our experiments is 200 nm. But the thickness of the residual layer Tc in microcracks is difficult to measure experimentally. Lacour et al [43] estimated that it was about one atomic layer thick for Au strips. To match the experimental value of G of 1058, the residual thickness of Au, Tc, needs to be 0.2 nm, from equation (4), which, on an average, is between one and two monolayers of Au (lattice constant of Au is ∼0.4 nm). It is quite interesting, and clearly demonstrated by our experiments for the first time, that such thin layers of Au can be generated on PDMS quite reversibly, especially because at that low film thickness, the Au layer is likely to be present in the form of nanoparticles, which would possibly conduct through hopping electron conduction. Our choice of 200 nm Au thickness is based on past studies where the Au thickness varied from 5 to 500 nm [36–38, 41–43]. In this work, we chose a mid-range value of 200 nm as the initial sensor design parameter for the thickness of the Au thin film resistor. The optimization of the thin film thickness for better sensor performance (i.e. sensitivity, effective operating range, stability, etc) will be conducted in our future work, which will also help us to understand the possible formation mechanisms of these microcracks.
4. Conclusions
In conclusion, we have demonstrated a simple yet highly sensitive pressure sensor based on the Au film resistor patterned on the PDMS thin membrane that is suitable for biosensing applications. The sensor demonstrated an extremely high sensitivity of 0.23/KPa, over a pressure range of 0–50 mm of Hg, resulting in a gauge factor of 1058, which are the best results reported so far for any flexible membrane-based sensor over that pressure range. The noise-limited pressure resolution and response time of the sensors have been measured to be 0.9 Pa and ∼200 ms, which are also the best values reported so far for these sensors. The sensor has been tested for reliability, and has been found to retain a similar response even after rigorous testing over a few weeks. The extremely high sensitivity of the sensor is attributed to the formation of microcracks, and has been correlated with the sensor parameters using a simple model, which predicts that the thickness of the Au film can reduce to an average thickness of one to two monolayers in the cracks, causing very high resistance changes.
Acknowledgments
We acknowledge financial support for this work from American Heart Association (AHA) Foundations (grant no 10SDG2600256) and from the National Science Foundation (grant nos ECCS-1029346 and ECCS-0846898). Facility usage permission from Dr Richard Webb of the Physics Department, University of South Carolina, is thankfully acknowledged. We also acknowledge help from Dr Jian Liu from the Medical University of South Carolina with the fabrication of shadow masks used in this work.












