Trans-Cyclooctene: Difference between revisions
→trans-Cyclooctene: tweak sectionlink |
m Substing templates: {{Format ISBN}}. See User:AnomieBOT/docs/TemplateSubster for info. |
||
| (48 intermediate revisions by 21 users not shown) | |||
| Line 1: | Line 1: | ||
| ⚫ | |||
{{chembox |
{{chembox |
||
| |
|Reference =<ref>{{cite web |url= http://www.sigmaaldrich.com/catalog/search/ProductDetail/ALDRICH/125482 |title= ''cis''-Cyclooctene |publisher= [[Sigma-Aldrich]] }}</ref> |
||
| |
|Name =''trans''-Cyclooctene |
||
| |
|ImageFile =Trans-Cyclooctene.png |
||
| |
|ImageSize = 344 px |
||
| |
|PIN =(''E'')-Cyclooctene |
||
| |
|OtherNames =''trans''-Cyclooctene |
||
|Section1={{Chembox Identifiers |
|Section1={{Chembox Identifiers |
||
| |
|ChemSpiderID = 10265272 |
||
| |
|InChI=1S/C8H14/c1-2-4-6-8-7-5-3-1/h1-2H,3-8H2/b2-1+ |
||
| |
|InChIKey = URYYVOIYTNXXBN-OWOJBTEDSA-N |
||
| |
|CASNo =931-89-5 |
||
| |
|PubChem =5463599 |
||
|ChEBI = 73156 |
|||
| ⚫ | |||
|EINECS = 213-245-5 |
|||
| ⚫ | |||
}} |
}} |
||
|Section2={{Chembox Properties |
|Section2={{Chembox Properties |
||
| |
|C=8 | H=14 |
||
| |
|Appearance = colorless liquid |
||
| |
|Density =0.848 g/mL |
||
| |
|MeltingPtC = -59 |
||
|BoilingPt = 143 °C (1 atm); 68-72 °C (100 torr)<ref name=vede1973/> |
|||
| BoilingPtC = 145 to 146 |
|||
| ⚫ | |||
| BoilingPt_notes = |
|||
| Solubility = |
|||
}} |
|||
|Section3={{Chembox Hazards |
|Section3={{Chembox Hazards |
||
|GHSPictograms = {{GHS02}}{{GHS08}} |
|||
| MainHazards = |
|||
|GHSSignalWord = Danger |
|||
| FlashPt = |
|||
| ⚫ | |||
| AutoignitionPt = |
|||
| ⚫ | |||
}} |
}} |
||
'''''trans''-Cyclooctene''' is a [[cyclic compound|cyclic]] [[hydrocarbon]] with the formula [–(CH<sub>2</sub>)<sub>6</sub>CH=CH–], where the two C–C single bonds adjacent to the double bond are on opposite sides of the latter's plane. It is a colorless liquid with a disagreeable odor. |
|||
'''Cyclooctene''' is a [[cycloalkene]] with an eight-membered ring. It is notable because it is the smallest cycloalkene that can exist as either the [[cis–trans isomerism|''cis''- or ''trans'']]-[[isomer]] with the ''cis''-isomer more common. Its most stable ''cis'' [[stereoisomer]] can adopt various conformations, the most stable one being shaped like a ribbon;<ref name="cis">{{cite journal |first1= Ulrich |last1= Neuenschwander |first2= Ive |last2= Hermans |doi= 10.1021/jo202176j |title= The Conformations of Cyclooctene: Consequences for Epoxidation Chemistry |journal= [[J. Org. Chem.]] |volume= 76 |issue= 24 |pages= 10236–10240 |year= 2011 }}</ref> its most stable ''trans''-conformer is shaped like the 8-carbon equivalent chair conformation of cyclohexane. |
|||
[[Cyclooctene]] is notable as the smallest cycloalkene that is readily isolated as its ''trans''-[[isomer]]. The ''cis''-isomer is much more stable;<ref name=neue2011>{{cite journal |first1= Ulrich |last1= Neuenschwander |first2= Ive |last2= Hermans |doi= 10.1021/jo202176j |title= The conformations of cyclooctene: Consequences for epoxidation chemistry |journal=[[Journal of Organic Chemistry]]|volume= 76 |issue= 24 |pages= 10236–10240 |year= 2011 |pmid= 22077196 }}</ref> the ring-strain energies being 16.7 and 7.4 kcal/mol, respectively.<ref name=walk2009>{{cite journal | doi=10.1021/ma801693q | title=The Living ROMP of ''trans''-Cyclooctene | year=2009 | last1=Walker | first1=Ron | last2=Conrad | first2=Rosemary M. | last3=Grubbs | first3=Robert H. | journal=[[Macromolecules (journal)|Macromolecules]] | volume=42 | issue=3 | pages=599–605 | pmid=20379393 | pmc=2850575 | bibcode=2009MaMol..42..599W}}</ref> |
|||
{| class="wikitable" |
|||
| ⚫ | |||
{| style="margin-left:auto;margin-right:auto;" |
|||
| ⚫ | |||
|- align="center" |
|- align="center" |
||
| ''cis''-Cyclooctene |
| [[cis-Cyclooctene|''cis''-Cyclooctene]]<br />in ''chair'' conformation ||(''R<sub>p</sub>'')-''trans''-Cyclooctene<br />in ''crown'' conformation |
||
|} |
|} |
||
{{clear-left}} |
{{clear-left}} |
||
A planar arrangement of the ring carbons would be too strained, and therefore the stable [[conformational isomer|conformations]] of the ''trans'' form have a bent (non-planar) ring. Computations indicate that the most stable "crown" conformation has the carbon atoms alternately above and below the plane of the ring.<ref name=selv2013/> A "half-chair" conformation, with about 6 kcal/mol higher energy, has carbons 2,3,5,6, and 8 on the same side of the plane of carbons 1,4, and 7.<ref name=selv2013/> |
|||
==''cis''-Cyclooctene== |
|||
{{confusing section|date=August 2016}} |
|||
All conformations of ''trans''-cyclooctene are [[chirality|chiral]] (specifically, what some call [[planar chirality#In chemistry|planar-chiral]]<ref>{{GoldBookRef |file= P04681 |title= Planar chirality}}</ref>) and the [[enantiomer]]s can be separated.<ref name=cope1963>{{cite journal | doi=10.1021/ja00903a049 | title=Molecular Asymmetry of Olefins. I. Resolution of ''trans''-Cyclooctene<sup>1-3</sup> | year=1963 | last1=Cope | first1=Arthur C. | last2=Ganellin | first2=C. R. | last3=Johnson | first3=H. W. | last4=Van Auken | first4=T. V. | last5=Winkler | first5=Hans J. S. | journal=[[Journal of the American Chemical Society]] | volume=85 | issue=20 | pages=3276–3279 }}</ref><ref name=cope1964>{{cite journal | doi=10.1021/ja01078a044 | title=Molecular Asymmetry of Olefins. II. The Absolute Configuration of ''trans''-Cyclooctene | year=1964 | last1=Cope | first1=Arthur C. | last2=Mehta | first2=Anil S. | journal=[[Journal of the American Chemical Society]] | volume=86 | issue=24 | pages=5626–5630 }}</ref><ref>{{cite encyclopedia|title=(−)-Dichloro(ethylene)(α-methylbenzylamine)platinum(II)|author=Steven D. Paget |encyclopedia= Encyclopedia of Reagents for Organic Synthesis|year=2001|publisher= John Wiley & Sons|doi=10.1002/047084289X.rd119|isbn=0-471-93623-5 }}</ref> In theory, conversion of between the enantiomers can be done, without breaking any bonds, by twisting the whole –CH=CH– group, rigidly, by 180 degrees. However, that entails passing one of its hydrogens through the crowded ring.<ref name=cope1963/> |
|||
''cis''-Cyclooctene (COE) is a substrate notoriously known for quite selectively forming the epoxide, as compared to other cycloalkenes, e.g. cyclohexene. Low amounts of radical by-products are found only. The reason for this behaviour is that allylic functionalization in ''cis''-cyclooctene is more difficult than for other cycloalkenes, because of almost orthogonal allylic C-H bonds. Therefore, if radicals are around, they rather form epoxide (via an addition-elimination mechanism) than to form allylic byproducts.<ref name="cis" /> It is used as an easily displaced [[ligand]] in [[organometallic chemistry]], e.g. [[chlorobis(cyclooctene)rhodium dimer]] and [[chlorobis(cyclooctene)iridium dimer]]. |
|||
==Preparation== |
|||
| ⚫ | |||
| ⚫ | ''trans''-Cyclooctene was first synthesized on a preparatory scale by [[Arthur C. Cope]] with a [[Hofmann elimination]] reaction of ''N,N,N''-trimethylcyclooctylammonium iodide.<ref name=cope1969>{{OrgSynth |first1= Arthur C. |last1= Cope |author1-link= Arthur C. Cope |first2= Robert D. |last2= Bach |year=1969 |title=''trans''-Cyclooctene |volume=49 |pages=39 |collvol=5 |collvolpages=315 |prep=CV5P0315}}</ref> The reaction gives a mixture of ''cis'' and ''trans'' isomers, and the ''trans'' isomer is selectively [[chemical trap|trapped]] as a [[Coordination complex|complex]] with [[silver nitrate]]. |
||
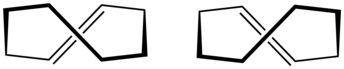
''trans''-Cyclooctene is the smallest cycloalkene in which the ''trans''-isomer is stable at room temperature. This is because ''trans''-cycloalkenes have a longer bridging distance between the two [[allylic|allylic carbons]] than their respective ''cis''-cycloalkenes. Eight carbons is the minimum ring size required to join them when they are ''trans'' without causing severe structural strain. The smaller rings of ''trans''-[[Cycloheptene]] and ''trans''-[[cyclohexene]] can exist, but they are very unstable at room temperature. ''trans''-Cyclooctene exists in a helical conformation with the carbon chain lying above the double bond on one side and below it on the other, leading to [[chirality]] (as depicted to the right). This type of chirality is defined as [[planar chirality#In chemistry|planar chirality]]. There are two [[enantiomer]]ic forms, [[atropisomer]]s resulting from restricted rotation about a single bond.<ref>{{GoldBookRef |file= P04681 |title= Planar chirality}}</ref> |
|||
Other methods exist where the ''trans'' isomer is synthesized from the ''cis'' isomer in several synthetic steps. For instance, it can be prepared in almost 100% yield by converting the ''cis'' isomer to [[1,2-epoxycyclooctane]] ("cyclooctene oxide") followed by reactions with [[lithium diphenylphosphide]] ({{chem|LiPPh|2}}) and with [[methyl iodide]] {{chem|CH|3|I}}. (Similar procedures can give ''{{chem name|cis'',''trans}}'' isomers of [[1,4-cyclooctadiene]]<!-- MP 208-209° --> and [[1,5-cyclooctadiene]]<!-- MP (mp 178-179°-->).<ref name=vede1973>{{cite journal | doi=10.1021/jo00946a024 | title=Phosphorus betaines derived from cycloheptene and cyclooctene oxides. Inversion of cyclooctene | year=1973 | last1=Vedejs | first1=Edwin | last2=Snoble | first2=Karel A. J. | last3=Fuchs | first3=Philip L. | journal=[[Journal of Organic Chemistry]] | volume=38 | issue=6 | pages=1178–1183 }}</ref> |
|||
| ⚫ | ''trans''-Cyclooctene was first synthesized on a preparatory scale by [[Arthur C. Cope]] with a [[Hofmann elimination]] reaction of ''N,N,N''-trimethylcyclooctylammonium iodide.<ref>{{OrgSynth |first1= Arthur C. |last1= Cope |author1-link= Arthur C. Cope |first2= Robert D. |last2= Bach |year=1969 |title=''trans''-Cyclooctene |volume=49 |pages=39 |collvol=5 |collvolpages=315 |prep=CV5P0315}}</ref> The reaction gives a mixture of ''cis'' and ''trans'' isomers, and the ''trans'' isomer is selectively [[chemical trap|trapped]] as a [[Coordination complex|complex]] with [[silver nitrate]]. |
||
| ⚫ | In addition, a [[photochemical]] method exists for the direct ''cis''–''trans'' isomerisation. Although this equilibrium strongly favours the more stable ''cis'' form, the reaction can be driven towards the ''trans'' form by trapping with silver ions.<ref name=swen1969>{{cite journal | doi=10.1021/jo01262a102 | title=Photoisomerization of ''cis''-cyclooctene to ''trans''-cyclooctene | year=1969 | last1=Swenton | first1=John S. | journal=[[Journal of Organic Chemistry]] | volume=34 | issue=10 | pages=3217–3218 }}</ref><ref name=royz2008>{{cite journal |title= A photochemical synthesis of functionalized ''trans''-cyclooctenes driven by metal complexation |first1= Maksim |last1= Royzen |first2= Glenn P. A. |last2= Yap |first3= Joseph M. |last3= Fox |journal= [[Journal of the American Chemical Society]]|year= 2008 |volume= 130 |issue= 12 |pages= 3760–3761 |doi= 10.1021/ja8001919 |pmid= 18321114 }}</ref> |
||
: |
|||
==Reactions== |
|||
| ⚫ | |||
Because of the higher internal strain on the double bond, the ''trans'' isomer is more reactive than the ''cis'' isomer and of typical unsaturated hydrocarbons. For instance, its double bond will rapidly [[addition reaction|add]] [[tetrazine]] and its derivatives.<ref name=selv2013>{{cite journal | doi=10.1016/j.cbpa.2013.07.031 | title=''trans''-Cyclooctene — A stable, voracious dienophile for bioorthogonal labeling | year=2013 | last1=Selvaraj | first1=Ramajeyam | last2=Fox | first2=Joseph M. | journal=[[Current Opinion in Chemical Biology]] | volume=17 | issue=5 | pages=753–760 | pmid=23978373 | pmc=3925366 }}</ref> The compound also readily polymerizes with a [[ruthenium]]-based initiator.<ref name=walk2009/> |
|||
==References== |
==References== |
||
| Line 59: | Line 62: | ||
{{Authority control}} |
{{Authority control}} |
||
[[Category:Cycloalkenes]] |
[[Category:Cycloalkenes]] |
||
[[Category:Foul-smelling chemicals]] |
|||
Latest revision as of 17:29, 30 September 2023
| |
| Names | |
|---|---|
| Preferred IUPAC name
(E)-Cyclooctene | |
| Other names
trans-Cyclooctene
| |
| Identifiers | |
3D model (JSmol)
|
|
| ChEBI | |
| ChemSpider | |
| EC Number |
|
PubChem CID
|
|
CompTox Dashboard (EPA)
|
|
| |
| |
| Properties | |
| C8H14 | |
| Molar mass | 110.200 g·mol−1 |
| Appearance | colorless liquid |
| Density | 0.848 g/mL |
| Melting point | −59 °C (−74 °F; 214 K) |
| Boiling point | 143 °C (1 atm); 68-72 °C (100 torr)[2] |
| Hazards | |
| GHS labelling: | |
 
| |
| Danger | |
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa).
| |
trans-Cyclooctene is a cyclic hydrocarbon with the formula [–(CH2)6CH=CH–], where the two C–C single bonds adjacent to the double bond are on opposite sides of the latter's plane. It is a colorless liquid with a disagreeable odor.
Cyclooctene is notable as the smallest cycloalkene that is readily isolated as its trans-isomer. The cis-isomer is much more stable;[3] the ring-strain energies being 16.7 and 7.4 kcal/mol, respectively.[4]
 |
|
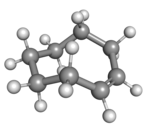
| cis-Cyclooctene in chair conformation |
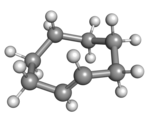
(Rp)-trans-Cyclooctene in crown conformation |
A planar arrangement of the ring carbons would be too strained, and therefore the stable conformations of the trans form have a bent (non-planar) ring. Computations indicate that the most stable "crown" conformation has the carbon atoms alternately above and below the plane of the ring.[5] A "half-chair" conformation, with about 6 kcal/mol higher energy, has carbons 2,3,5,6, and 8 on the same side of the plane of carbons 1,4, and 7.[5]
All conformations of trans-cyclooctene are chiral (specifically, what some call planar-chiral[6]) and the enantiomers can be separated.[7][8][9] In theory, conversion of between the enantiomers can be done, without breaking any bonds, by twisting the whole –CH=CH– group, rigidly, by 180 degrees. However, that entails passing one of its hydrogens through the crowded ring.[7]
Preparation
[edit]trans-Cyclooctene was first synthesized on a preparatory scale by Arthur C. Cope with a Hofmann elimination reaction of N,N,N-trimethylcyclooctylammonium iodide.[10] The reaction gives a mixture of cis and trans isomers, and the trans isomer is selectively trapped as a complex with silver nitrate.
Other methods exist where the trans isomer is synthesized from the cis isomer in several synthetic steps. For instance, it can be prepared in almost 100% yield by converting the cis isomer to 1,2-epoxycyclooctane ("cyclooctene oxide") followed by reactions with lithium diphenylphosphide (LiPPh
2) and with methyl iodide CH
3I. (Similar procedures can give cis,trans isomers of 1,4-cyclooctadiene and 1,5-cyclooctadiene).[2]
In addition, a photochemical method exists for the direct cis–trans isomerisation. Although this equilibrium strongly favours the more stable cis form, the reaction can be driven towards the trans form by trapping with silver ions.[11][12]
Reactions
[edit]Because of the higher internal strain on the double bond, the trans isomer is more reactive than the cis isomer and of typical unsaturated hydrocarbons. For instance, its double bond will rapidly add tetrazine and its derivatives.[5] The compound also readily polymerizes with a ruthenium-based initiator.[4]
References
[edit]- ^ "cis-Cyclooctene". Sigma-Aldrich.
- ^ a b Vedejs, Edwin; Snoble, Karel A. J.; Fuchs, Philip L. (1973). "Phosphorus betaines derived from cycloheptene and cyclooctene oxides. Inversion of cyclooctene". Journal of Organic Chemistry. 38 (6): 1178–1183. doi:10.1021/jo00946a024.
- ^ Neuenschwander, Ulrich; Hermans, Ive (2011). "The conformations of cyclooctene: Consequences for epoxidation chemistry". Journal of Organic Chemistry. 76 (24): 10236–10240. doi:10.1021/jo202176j. PMID 22077196.
- ^ a b Walker, Ron; Conrad, Rosemary M.; Grubbs, Robert H. (2009). "The Living ROMP of trans-Cyclooctene". Macromolecules. 42 (3): 599–605. Bibcode:2009MaMol..42..599W. doi:10.1021/ma801693q. PMC 2850575. PMID 20379393.
- ^ a b c Selvaraj, Ramajeyam; Fox, Joseph M. (2013). "trans-Cyclooctene — A stable, voracious dienophile for bioorthogonal labeling". Current Opinion in Chemical Biology. 17 (5): 753–760. doi:10.1016/j.cbpa.2013.07.031. PMC 3925366. PMID 23978373.
- ^ IUPAC, Compendium of Chemical Terminology, 2nd ed. (the "Gold Book") (1997). Online corrected version: (2006–) "Planar chirality". doi:10.1351/goldbook.P04681
- ^ a b Cope, Arthur C.; Ganellin, C. R.; Johnson, H. W.; Van Auken, T. V.; Winkler, Hans J. S. (1963). "Molecular Asymmetry of Olefins. I. Resolution of trans-Cyclooctene1-3". Journal of the American Chemical Society. 85 (20): 3276–3279. doi:10.1021/ja00903a049.
- ^ Cope, Arthur C.; Mehta, Anil S. (1964). "Molecular Asymmetry of Olefins. II. The Absolute Configuration of trans-Cyclooctene". Journal of the American Chemical Society. 86 (24): 5626–5630. doi:10.1021/ja01078a044.
- ^ Steven D. Paget (2001). "(−)-Dichloro(ethylene)(α-methylbenzylamine)platinum(II)". Encyclopedia of Reagents for Organic Synthesis. John Wiley & Sons. doi:10.1002/047084289X.rd119. ISBN 0-471-93623-5.
- ^ Cope, Arthur C.; Bach, Robert D. (1969). "trans-Cyclooctene". Organic Syntheses. 49: 39; Collected Volumes, vol. 5, p. 315.
- ^ Swenton, John S. (1969). "Photoisomerization of cis-cyclooctene to trans-cyclooctene". Journal of Organic Chemistry. 34 (10): 3217–3218. doi:10.1021/jo01262a102.
- ^ Royzen, Maksim; Yap, Glenn P. A.; Fox, Joseph M. (2008). "A photochemical synthesis of functionalized trans-cyclooctenes driven by metal complexation". Journal of the American Chemical Society. 130 (12): 3760–3761. doi:10.1021/ja8001919. PMID 18321114.
