Silver sulfate: Difference between revisions
Appearance
Content deleted Content added
No edit summary |
|||
| (5 intermediate revisions by 4 users not shown) | |||
| Line 87: | Line 87: | ||
==Preparation and structure== |
==Preparation and structure== |
||
Silver sulfate precipitates as a solid when an aqueous solution of [[silver nitrate]] is treated with [[sulfuric acid]]: |
Silver sulfate precipitates as a solid when an aqueous solution of [[silver nitrate]] is treated with [[sulfuric acid]]: |
||
:{{chem2 | 2 AgNO3 + H2SO4 -> Ag2SO4 + 2 HNO3 }} |
|||
:2{{nbsp}}AgNO<sub>3</sub> + H<sub>2</sub>SO<sub>4</sub> → Ag<sub>2</sub>SO<sub>4</sub> + 2{{nbsp}}HNO<sub>3</sub> |
|||
It is purified by recrystallization from concentrated sulfuric acid, a step that expels traces of nitrate.<ref>{{cite book|author1=O. Glemser |
It is purified by recrystallization from concentrated sulfuric acid, a step that expels traces of nitrate.<ref>{{cite book |author1=O. Glemser |title=Handbook of Preparative Inorganic Chemistry, 2nd Ed. |author2=R. Sauer |publisher=Academic Press |year=1963 |editor=G. Brauer |volume=2pages=1042 |place=NY, NY |chapter=Silver Sulfate}}</ref> |
||
Silver sulfate and anhydrous [[sodium sulfate]] adopt the same structure.<ref>{{cite journal |doi=10.1524/zkri.1932.82.1.161 |title=Note on the Crystal Structure of Silver Sulphate, Ag<sub>2</sub>SO<sub>4</sub> |year=1932 |last1=Zachariasen |first1=W. H. |journal=Zeitschrift für Kristallographie - Crystalline Materials |volume=82 |issue=1–6 |pages=161–162 |s2cid=101362527 }}</ref> |
Silver sulfate and anhydrous [[sodium sulfate]] adopt the same structure.<ref>{{cite journal |doi=10.1524/zkri.1932.82.1.161 |title=Note on the Crystal Structure of Silver Sulphate, Ag<sub>2</sub>SO<sub>4</sub> |year=1932 |last1=Zachariasen |first1=W. H. |journal=Zeitschrift für Kristallographie - Crystalline Materials |volume=82 |issue=1–6 |pages=161–162 |s2cid=101362527 }}</ref> |
||
| Line 94: | Line 94: | ||
The synthesis of '''silver(II) sulfate''' (AgSO<sub>4</sub>) with a divalent silver ion instead of a monovalent silver ion was first reported in 2010<ref>{{Cite journal| doi = 10.1002/anie.200906863| pmid = 20084660| year = 2010| last1 = Malinowski | first1 = P.| last2 = Derzsi | first2 = M.| last3 = Mazej | first3 = Z.| last4 = Jagličić | first4 = Z.| last5 = Gaweł | first5 = B.| last6 = Lasocha | first6 = W.| last7 = Grochala | first7 = W.| title = Ag(II)SO(4): A Genuine Sulfate of Divalent Silver with Anomalously Strong One-Dimensional Antiferromagnetic Interactions.| volume = 49| issue = 9| pages = 1683–1686| journal = Angewandte Chemie International Edition in English | doi-access = free}}</ref> by adding [[sulfuric acid]] to [[silver(II) fluoride]] ([[Hydrogen fluoride|HF]] escapes). It is a black solid that decomposes exothermically at 120 °C with evolution of oxygen and the formation of the [[pyrosulfate]]. |
The synthesis of '''silver(II) sulfate''' (AgSO<sub>4</sub>) with a divalent silver ion instead of a monovalent silver ion was first reported in 2010<ref>{{Cite journal| doi = 10.1002/anie.200906863| pmid = 20084660| year = 2010| last1 = Malinowski | first1 = P.| last2 = Derzsi | first2 = M.| last3 = Mazej | first3 = Z.| last4 = Jagličić | first4 = Z.| last5 = Gaweł | first5 = B.| last6 = Lasocha | first6 = W.| last7 = Grochala | first7 = W.| title = Ag(II)SO(4): A Genuine Sulfate of Divalent Silver with Anomalously Strong One-Dimensional Antiferromagnetic Interactions.| volume = 49| issue = 9| pages = 1683–1686| journal = Angewandte Chemie International Edition in English | doi-access = free}}</ref> by adding [[sulfuric acid]] to [[silver(II) fluoride]] ([[Hydrogen fluoride|HF]] escapes). It is a black solid that decomposes exothermically at 120 °C with evolution of oxygen and the formation of the [[pyrosulfate]]. |
||
:{{chem2 | AgF2 + H2SO4 -> AgSO4 + 2 HF }} |
|||
AgF<sub>2</sub>+H<sub>2</sub>SO<sub>4</sub> →AgSO<sub>4</sub> +2HF |
|||
:{{chem2 | 4 AgSO4 -> 2 Ag2S2O7 + O2 }} |
|||
==References== |
==References== |
||
| Line 104: | Line 105: | ||
[[Category:Silver compounds]] |
[[Category:Silver compounds]] |
||
[[Category:Sulfates]] |
[[Category:Sulfates]] |
||
{{inorganic-compound-stub}} |
|||
Latest revision as of 18:48, 10 April 2024
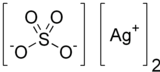
| |

| |

| |
| Names | |
|---|---|
| IUPAC name
Silver(I) sulfate
| |
| Other names
Disilver sulfate
Argentous sulfate | |
| Identifiers | |
3D model (JSmol)
|
|
| ChemSpider | |
| ECHA InfoCard | 100.030.581 |
| EC Number |
|
PubChem CID
|
|
| UNII | |
| UN number | 3077 |
CompTox Dashboard (EPA)
|
|
| |
| |
| Properties | |
| Ag2SO4 | |
| Molar mass | 311.79 g·mol−1 |
| Appearance | Colorless solid |
| Odor | Odorless |
| Density | 5.45 g/cm3 (25 °C) 4.84 g/cm3 (660 °C)[1] |
| Melting point | 652.2–660 °C (1,206.0–1,220.0 °F; 925.4–933.1 K)[1][5] |
| Boiling point | 1,085 °C (1,985 °F; 1,358 K)[3][5] decomposition |
| 0.57 g/100 mL (0 °C) 0.69 g/100 mL (10 °C) 0.83 g/100 mL (25 °C) 0.96 g/100 mL (40 °C) 1.33 g/100 mL (100 °C)[2] | |
Solubility product (Ksp)
|
1.2·10−5[1] |
| Solubility | Dissolves in aq. acids, alcohols, acetone, ether, acetates, amides[2] Insoluble in ethanol[3] |
| Solubility in sulfuric acid | 8.4498 g/L (0.1 molH2SO4/LH2O)[2] 25.44 g/100 g (13 °C) 31.56 g/100 g (24.5 °C) 127.01 g/100 g (96 °C)[3] |
| Solubility in ethanol | 7.109 g/L (0.5 nEtOH/H2O)[2] |
| Solubility in acetic acid | 7.857 g/L (0.5 nAcOH/H2O)[2] |
| −9.29·10−5 cm3/mol[1] | |
Refractive index (nD)
|
nα = 1.756 nβ = 1.775 nγ = 1.782[4] |
| Structure | |
| Orthorhombic, oF56[4] | |
| Fddd, No. 70[4] | |
| 2/m 2/m 2/m[4] | |
a = 10.2699(5) Å, b = 12.7069(7) Å, c = 5.8181(3) Å[4] α = 90°, β = 90°, γ = 90°
| |
| Thermochemistry | |
Heat capacity (C)
|
131.4 J/mol·K[1] |
Std molar
entropy (S⦵298) |
200.4 J/mol·K [1] |
Std enthalpy of
formation (ΔfH⦵298) |
−715.9 kJ/mol[1] |
Gibbs free energy (ΔfG⦵)
|
−618.4 kJ/mol [1] |
| Hazards | |
| GHS labelling: | |
  [6] [6]
| |
| Danger | |
| H318, H410[6] | |
| P273, P280, P305+P351+P338, P501[6] | |
| NFPA 704 (fire diamond) | |
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa).
| |
Silver sulfate is the inorganic compound with the formula Ag2SO4. It is a white solid with low solubility in water.
Preparation and structure
[edit]Silver sulfate precipitates as a solid when an aqueous solution of silver nitrate is treated with sulfuric acid:
- 2 AgNO3 + H2SO4 → Ag2SO4 + 2 HNO3
It is purified by recrystallization from concentrated sulfuric acid, a step that expels traces of nitrate.[7] Silver sulfate and anhydrous sodium sulfate adopt the same structure.[8]
Silver(II) sulfate
[edit]The synthesis of silver(II) sulfate (AgSO4) with a divalent silver ion instead of a monovalent silver ion was first reported in 2010[9] by adding sulfuric acid to silver(II) fluoride (HF escapes). It is a black solid that decomposes exothermically at 120 °C with evolution of oxygen and the formation of the pyrosulfate.
- AgF2 + H2SO4 → AgSO4 + 2 HF
- 4 AgSO4 → 2 Ag2S2O7 + O2
References
[edit]- ^ a b c d e f g h Lide, David R., ed. (2009). CRC Handbook of Chemistry and Physics (90th ed.). Boca Raton, Florida: CRC Press. ISBN 978-1-4200-9084-0.
- ^ a b c d e Seidell, Atherton; Linke, William F. (1919). Solubilities of Inorganic and Organic Compounds (2nd ed.). New York: D. Van Nostrand Company. pp. 622–623.
- ^ a b c Anatolievich, Kiper Ruslan. "silver sulfate". Retrieved 2014-07-19.
- ^ a b c d e Morris, Marlene C.; McMurdie, Howard F.; Evans, Eloise H.; Paretzkin, Boris; Groot, Johan H. de; Hubbard, Camden R.; Carmel, Simon J. (June 1976). "13". Standard X-ray Diffraction Powder Patterns. Vol. 25. Washington: Institute for Materials Research National Bureau of Standards.
- ^ a b c "MSDS of Silver sulfate". Fisher Scientific, Inc. Retrieved 2014-07-19.
- ^ a b c Sigma-Aldrich Co., Silver sulfate. Retrieved on 2014-07-19.
- ^ O. Glemser; R. Sauer (1963). "Silver Sulfate". In G. Brauer (ed.). Handbook of Preparative Inorganic Chemistry, 2nd Ed. Vol. 2pages=1042. NY, NY: Academic Press.
- ^ Zachariasen, W. H. (1932). "Note on the Crystal Structure of Silver Sulphate, Ag2SO4". Zeitschrift für Kristallographie - Crystalline Materials. 82 (1–6): 161–162. doi:10.1524/zkri.1932.82.1.161. S2CID 101362527.
- ^ Malinowski, P.; Derzsi, M.; Mazej, Z.; Jagličić, Z.; Gaweł, B.; Lasocha, W.; Grochala, W. (2010). "Ag(II)SO(4): A Genuine Sulfate of Divalent Silver with Anomalously Strong One-Dimensional Antiferromagnetic Interactions". Angewandte Chemie International Edition in English. 49 (9): 1683–1686. doi:10.1002/anie.200906863. PMID 20084660.

