Leukodystrophy: Difference between revisions
Ozzie10aaaa (talk | contribs) m Cleaned up using AutoEd |
Citation bot (talk | contribs) Alter: date, title. Add: chapter, doi-access, authors 1-1. Removed URL that duplicated identifier. Removed parameters. Some additions/deletions were parameter name changes. | Use this bot. Report bugs. | Suggested by Headbomb | Category:CS1 errors: dates | #UCB_Category 3/126 |
||
| (38 intermediate revisions by 17 users not shown) | |||
| Line 5: | Line 5: | ||
| synonyms = |
| synonyms = |
||
|image= Adrenoleukodystrophy.jpg |
|image= Adrenoleukodystrophy.jpg |
||
|caption= [[Magnetic resonance imaging#T1 and T2|T2 weighted]] [[transverse plane|axial]] scan at the level of the [[caudate nucleus|caudate]] heads demonstrates marked loss of posterior [[white matter]], with reduced volume and increased signal intensity. The anterior white matter is spared. Features are consistent with [[Sex linkage|X-linked]] [[adrenoleukodystrophy]]. |
|caption= [[Magnetic resonance imaging#T1 and T2|T2 weighted]] [[transverse plane|axial]] scan of a human brain at the level of the [[caudate nucleus|caudate]] heads demonstrates marked loss of posterior [[white matter]], with reduced volume and increased signal intensity. The anterior white matter is spared. Features are consistent with [[Sex linkage|X-linked]] [[adrenoleukodystrophy]]. |
||
| pronounce = |
| pronounce = |
||
| field = |
| field = |
||
| Line 24: | Line 24: | ||
| deaths = |
| deaths = |
||
}} |
}} |
||
'''Leukodystrophies''' are a group of usually inherited disorders characterized by [[neurodegeneration|degeneration]] of the [[white matter]] in the |
'''Leukodystrophies''' are a group of, usually, inherited disorders, characterized by [[neurodegeneration|degeneration]] of the [[white matter]] in the brain.<ref name="SachdevKeshavan2010">{{cite book|last1=Sachdev|first1=Perminder S.|last2=Keshavan|first2=Matcheri S.|title=Secondary Schizophrenia|url=https://books.google.com/books?id=44_zy35zFnIC&pg=PA241|access-date=15 August 2011|date=2010-03-15|publisher=Cambridge University Press|isbn=978-0-521-85697-3|pages=241–}}</ref> The word ''leukodystrophy'' comes from the [[Greek language|Greek]] roots ''leuko'', "white", ''dys'', "abnormal" and ''troph'', "growth". The leukodystrophies are caused by imperfect growth or development of the [[glial cells]] which produce the [[myelin|myelin sheath]], the fatty insulating covering around [[axon|nerve fibers]].<ref>{{Source-attribution|sentence=yes|"[https://www.ninds.nih.gov/Disorders/All-Disorders/Leukodystrophy-Information-Page Leukodystrophy Information Page]". National Institute of Neurological Disorders and Stroke. 25 May 2017. Retrieved 18 March 2018.}}</ref> Leukodystrophies may be classified as hypomyelinating or [[demyelinating diseases]], respectively, depending on whether the damage is present before birth or occurs after. While all leukodystrophies are the result of genetic mutations,<ref>{{Cite web |title=Leukodystrophy |url=https://www.ninds.nih.gov/health-information/disorders/leukodystrophy |access-date=2024-04-13 |website=www.ninds.nih.gov |language=en}}</ref> other demyelinating disorders have an [[autoimmune]], [[Infection|infectious]], or [[Metabolic disorder|metabolic]] etiology.<ref>{{Cite journal |last1=Coggan |first1=Jay S. |last2=Bittner |first2=Stefan |last3=Stiefel |first3=Klaus M. |last4=Meuth |first4=Sven G. |last5=Prescott |first5=Steven A. |date=September 2015 |title=Physiological Dynamics in Demyelinating Diseases: Unraveling Complex Relationships through Computer Modeling |journal=International Journal of Molecular Sciences |language=en |volume=16 |issue=9 |pages=21215–21236 |doi=10.3390/ijms160921215 |doi-access=free |issn=1422-0067 |pmc=4613250 |pmid=26370960}}</ref> |
||
When damage occurs to white matter, immune responses can lead to inflammation in the central nervous system (CNS), along with loss of myelin. The degeneration of white matter can be seen in an [[magnetic resonance imaging|MRI scan]] and used to diagnose leukodystrophy. Leukodystrophy is characterized by specific symptoms including decreased motor function, [[muscle rigidity]], and eventual degeneration of sight and hearing. While the disease is fatal, the age of onset is a key factor, as infants have a typical life expectancy of 2–8 years, while adults typically live more than a decade after onset. Treatment options are limited, although [[hematopoietic stem cell transplantation]]s using [[bone marrow]] or [[cord blood]] seem to help in certain types while further research is being done. |
When damage occurs to white matter, subsequent immune responses can lead to inflammation in the central nervous system (CNS), along with the loss of myelin. The degeneration of white matter can be seen in an [[magnetic resonance imaging|MRI scan]] and is used to diagnose leukodystrophy. Leukodystrophy is characterized by specific symptoms, including decreased motor function, [[muscle rigidity]], and eventual degeneration of sight and hearing. While the disease is fatal, the age of onset is a key factor, as infants have a typical life expectancy of 2–8 years, while adults typically live more than a decade after onset. Treatment options are limited, although [[hematopoietic stem cell transplantation]]s using [[bone marrow]] or [[cord blood]] seem to help in certain leukodystrophy types, while further research is being done. |
||
The combined incidence of the leukodystrophies is estimated at 1 in 7,600.<ref>{{cite journal|last1=Bonkowsky|first1=Joshua|title=The burden of inherited leukodystrophies in children|journal=Neurology|date=Aug 24, 2010|volume=75|issue=8|pages=718–725|doi=10.1212/WNL.0b013e3181eee46b|pmid=20660364|pmc=2931652}}</ref> The majority of types involve the inheritance of an [[X-linked recessive]], or [[X-linked dominant]] trait, while others, although involving a defective gene, are the result of [[spontaneous mutation]] rather than [[genetic inheritance]]. |
The combined incidence of the leukodystrophies is estimated at 1 in 7,600.<ref>{{cite journal|last1=Bonkowsky|first1=Joshua|title=The burden of inherited leukodystrophies in children|journal=Neurology|date=Aug 24, 2010|volume=75|issue=8|pages=718–725|doi=10.1212/WNL.0b013e3181eee46b|pmid=20660364|pmc=2931652}}</ref> The majority of types involve the inheritance of an [[X-linked recessive]], or [[X-linked dominant]] trait, while others, although involving a defective gene, are the result of [[spontaneous mutation]] rather than [[genetic inheritance]]. |
||
| Line 33: | Line 33: | ||
Some specific symptoms vary from one type of leukodystrophy to the next, but the vast majority of symptoms are shared as the causes for the disease generally have the same effects. Symptoms are dependent on the age of onset, which is predominantly in infancy and early childhood, although the exact time of onset may be difficult to determine. [[Hyperirritability]] and [[hypersensitivity]] to the environment are common, as well as some tell-tale physical signs including [[muscle rigidity]] and a backwards-bent head.<ref name="Krabbe disease">{{cite journal|last1=Graziano|first1=AC|last2=Cardile|first2=V|title=History, genetic, and recent advances on Krabbe disease|journal=Gene|date=26 September 2014|volume=555|issue=1|pages=2–13|pmid=25260228|doi=10.1016/j.gene.2014.09.046}}</ref> Botox therapy is often used to treat patients with spasticity.<ref>{{cite journal | pmc = 161748 | page=240 | volume=28 | issue=3 | journal=Journal of Psychiatry and Neuroscience | title=Tardive dystonia and its treatment| year=2003 | last1=Rosebush | first1=P. I. }}</ref> Juvenile and adult onsets display similar symptoms including a decrease or loss in hearing and vision. While children do experience optic and auditory degeneration, the course of the disease is usually too rapid, causing death relatively quickly, whereas adults may live with these conditions for many years. In children, spastic activity often precedes progressive [[ataxia]] and rapid cognitive deterioration which has been described as [[mental retardation]].<ref name="Chinese article">{{cite journal|last1=Liu|first1=Y|last2=Zou|first2=L|last3=Meng|first3=Y|last4=Zhang|first4=Y|last5=Shi|first5=X|last6=Ju|first6=J|last7=Yang|first7=G|last8=Hu|first8=L|last9=Chen|first9=X|title=[A family with two children diagnosed with aspartylglucosaminuria-case report and literature review].|journal=Zhonghua Er Za Zhi|date=June 2014|volume=52|issue=6|pages=455–9|pmid=25190167}}</ref> [[Epilepsy]] is commonplace for patients of all ages.<ref name="Spanish population">{{cite journal|last1=Turon-Vinas|first1=E|last2=Pineda|first2=M|last3=Cusi|first3=V|last4=Lopez-Laso|first4=E|last5=Del Pozo|first5=RL|last6=Gutierez-Solana|first6=LG|last7=Moreno|first7=DC|last8=Sierra-Corcoles|first8=C|last9=Olabarrieta-Hoyos|first9=N|last10=Madruga-Garrido|first10=M|last11=Aguirre-Rodriguez|first11=J|last12=Gonzalez-Alvarez|first12=V|last13=O'Callaghan|first13=M|last14=Muchart|first14=J|last15=Armstrong-Moron|first15=J|title=Vanishing white matter disease in a Spanish population.|journal=J Cent Nerv Syst Dis.|date=13 July 2014|volume=6|pages=59–68|pmid=25089094|doi=10.4137/JCNSD.S13540|pmc=4116383}}</ref> More progressed patients show weakness in [[deglutition]], leading to spastic coughing fits due to inhaled saliva. Classic symptomatic progression of juvenile [[X-linked adrenoleukodystrophy]] is shown in the 1992 film, ''[[Lorenzo's Oil]]''.<ref name="Lorenzos Oil">{{cite web|url=https://www.forbes.com/sites/ritarubin/2016/03/13/lorenzos-oil-could-not-cure-lorenzo-but-newborn-screening-is-expected-to-save-others-from-his-fate/|title=Forbes.com: Lorenzo's Oil Could Not Cure Lorenzo, But Newborn Screening Is Expected To Save Others From His Fate|last=Rubin|first=Rita|date=March 13, 2016|website=Forbes.com|access-date=July 31, 2018}}.</ref> |
Some specific symptoms vary from one type of leukodystrophy to the next, but the vast majority of symptoms are shared as the causes for the disease generally have the same effects. Symptoms are dependent on the age of onset, which is predominantly in infancy and early childhood, although the exact time of onset may be difficult to determine. [[Hyperirritability]] and [[hypersensitivity]] to the environment are common, as well as some tell-tale physical signs including [[muscle rigidity]] and a backwards-bent head.<ref name="Krabbe disease">{{cite journal|last1=Graziano|first1=AC|last2=Cardile|first2=V|title=History, genetic, and recent advances on Krabbe disease|journal=Gene|date=26 September 2014|volume=555|issue=1|pages=2–13|pmid=25260228|doi=10.1016/j.gene.2014.09.046}}</ref> Botox therapy is often used to treat patients with spasticity.<ref>{{cite journal | pmc = 161748 | page=240 | volume=28 | issue=3 | journal=Journal of Psychiatry and Neuroscience | title=Tardive dystonia and its treatment| year=2003 | last1=Rosebush | first1=P. I. }}</ref> Juvenile and adult onsets display similar symptoms including a decrease or loss in hearing and vision. While children do experience optic and auditory degeneration, the course of the disease is usually too rapid, causing death relatively quickly, whereas adults may live with these conditions for many years. In children, spastic activity often precedes progressive [[ataxia]] and rapid cognitive deterioration which has been described as [[mental retardation]].<ref name="Chinese article">{{cite journal|last1=Liu|first1=Y|last2=Zou|first2=L|last3=Meng|first3=Y|last4=Zhang|first4=Y|last5=Shi|first5=X|last6=Ju|first6=J|last7=Yang|first7=G|last8=Hu|first8=L|last9=Chen|first9=X|title=[A family with two children diagnosed with aspartylglucosaminuria-case report and literature review].|journal=Zhonghua Er Za Zhi|date=June 2014|volume=52|issue=6|pages=455–9|pmid=25190167}}</ref> [[Epilepsy]] is commonplace for patients of all ages.<ref name="Spanish population">{{cite journal|last1=Turon-Vinas|first1=E|last2=Pineda|first2=M|last3=Cusi|first3=V|last4=Lopez-Laso|first4=E|last5=Del Pozo|first5=RL|last6=Gutierez-Solana|first6=LG|last7=Moreno|first7=DC|last8=Sierra-Corcoles|first8=C|last9=Olabarrieta-Hoyos|first9=N|last10=Madruga-Garrido|first10=M|last11=Aguirre-Rodriguez|first11=J|last12=Gonzalez-Alvarez|first12=V|last13=O'Callaghan|first13=M|last14=Muchart|first14=J|last15=Armstrong-Moron|first15=J|title=Vanishing white matter disease in a Spanish population.|journal=J Cent Nerv Syst Dis.|date=13 July 2014|volume=6|pages=59–68|pmid=25089094|doi=10.4137/JCNSD.S13540|pmc=4116383}}</ref> More progressed patients show weakness in [[deglutition]], leading to spastic coughing fits due to inhaled saliva. Classic symptomatic progression of juvenile [[X-linked adrenoleukodystrophy]] is shown in the 1992 film, ''[[Lorenzo's Oil]]''.<ref name="Lorenzos Oil">{{cite web|url=https://www.forbes.com/sites/ritarubin/2016/03/13/lorenzos-oil-could-not-cure-lorenzo-but-newborn-screening-is-expected-to-save-others-from-his-fate/|title=Forbes.com: Lorenzo's Oil Could Not Cure Lorenzo, But Newborn Screening Is Expected To Save Others From His Fate|last=Rubin|first=Rita|date=March 13, 2016|website=Forbes.com|access-date=July 31, 2018}}.</ref> |
||
Course and timetable are dependent on the age of onset with infants showing a lifespan of 2–8 years, juveniles 2–10 years and adults typically 10+ years. Adults typically see an extended period of stability followed by a decline to a [[vegetative state]] and death.<ref name="Krabbe disease" /> While treatments do exist, most are in the experimental phase and can only promise a halt in the progression of symptoms, although some gene therapies have shown some symptomatic improvement.<ref name=Biffi/> The debilitating course of the disease has led to numerous philosophical and ethical arguments over experimental clinical trials, |
Course and timetable are dependent on the age of onset with infants showing a lifespan of 2–8 years, juveniles 2–10 years and adults typically 10+ years. Adults typically see an extended period of stability followed by a decline to a [[vegetative state]] and death.<ref name="Krabbe disease" /> While treatments do exist, most are in the experimental phase and can only promise a halt in the progression of symptoms, although some gene therapies have shown some symptomatic improvement.<ref name=Biffi/> The debilitating course of the disease has led to numerous philosophical and ethical arguments over experimental clinical trials, patients' rights and [[physician-assisted suicide]].<ref name=Ethics>{{cite journal|last1=Duchange|first1=N|last2=Darguy|first2=S|last3=d'Audiffret|first3=D|last4=Callies|first4=I|last5=Lapointe|first5=AS|last6=Loeve|first6=B|last7=Boespflug-Tanguy|first7=O|last8=Moutel|first8=G|title=Ethical management in the constitution of a European database for leukodystrophies rare diseases.|journal=Eur J Paediatr Neurol|date=18 September 2014|volume=18|issue=5|pages=597–603|pmid=24786336|doi=10.1016/j.ejpn.2014.04.002|s2cid=31385905|url=http://www.hal.inserm.fr/inserm-00995366/document}}</ref> |
||
==Causes== |
==Causes== |
||
While the more specific underlying causes of leukodystrophy are dependent upon the type, there are |
While the more specific underlying causes of leukodystrophy are dependent upon the type, there are common pathophysiological patterns that can be seen amongst all types. First and foremost, leukodystrophy is a neurodegenerative disease that is always the result of both impairment and maintenance of [[myelin]] sheaths surrounding neuronal [[axons]] in the [[central nervous system]] as the result of a [[genetic mutation]].<ref>{{cite journal|last1=Yang|first1=Edward|last2=Prabhu|first2=Sanjay P.|title=Imaging manifestations of the leukodystrophies, inherited disorders of white matter.|journal=Radiologic Clinics of North America|date=March 5, 2014|volume=52|issue=2|pages=279–319|doi=10.1016/j.rcl.2013.11.008|pmid=24582341}}</ref> Myelin is a fatty white substance that acts as an [[electrical insulator]] and coats axons in order to speed up impulses (i.e., [[action potentials]]) traveling down the axon. Thus, the natural result of a loss of this substance is decreased efficiency in impulse propagation. As myelin is produced by [[oligodendrocytes]] (a type of [[glial cell]]) in the central nervous system, an easy place to look for the cause is a [[mutation]] or malfunctioning of these cells and in other glial cells.{{citation needed|date=September 2020}} |
||
===Genetic influence=== |
===Genetic influence=== |
||
[[File:Autorecessive.svg|thumb|Autorecessive Inheritance Pattern]] |
[[File:Autorecessive.svg|thumb|Autorecessive Inheritance Pattern]] |
||
Inherited forms of leukodystrophy are usually the result of an [[autosomal recessive]] inheritance pattern, although dominant inheritance patterns are not unheard of, as in the case of adult-onset leukodystrophy.<ref name="Autosomal Dominant Leukodystrophy">{{cite journal|last1=Lin|first1=Shu-Ting|last2=Ptacek|first2=Louis J.|last3=Fu|first3=Ying-Hui|title=Adult-Onset Autosomal Dominant Leukodystrophy: Linking Nuclear Envelope to Myelin|journal=The Journal of Neuroscience|date=January 26, 2011|volume=31|issue=4|pages=1163–1166|doi=10.1523/jneurosci.5994-10.2011|pmid=21273400|pmc=3078713}}</ref> This means that the affected [[allele]] is carried on an [[autosomal]], or non-sex, chromosome and is masked by the dominant, unaffected [[phenotype]]. In other words, for an individual to inherit the leukodystrophy phenotype, he or she must carry two of the recessive, mutant alleles. [[Krabbe disease]] and [[metachromatic leukodystrophy]] (MLD) are two of such type. MLD is found on human [[chromosome 22]] at position q13.31.<ref name="Metachromatic - Chromosome #">{{cite journal|last1=Coulter-Mackie|first1=MB|last2=Rip|first2=J|last3=Ludman|first3=MD|last4=Beis|first4=J|last5=Cole|first5=DEC|title=Metachromatic leucodystrophy (MLD) in a patient with a constitutional ring chromosome 22|journal=Journal of Medical Genetics|date=October 1995|volume=32|issue=10|pmc=1051701|pmid=8558556|pages=787–91|doi=10.1136/jmg.32.10.787}}</ref> Another type of inherited leukodystrophy is [[X-linked adrenoleukodystrophy]] (X-ALD). As its name implies, this type of leukodystrophy is the result of a mutation found on the [[X-chromosome]]. It is also carried in a recessive pattern. The X chromosome is a [[sex chromosome]], and since women have two "chances" of acquiring a normal X chromosome (one maternal x, one paternal x), and males only one chance (one maternal x), this disease is more likely to be seen in males than in females. The mutation resulting in adult-onset leukodystrophy is mapped at 5q23.<ref name="Autosomal Dominant Leukodystrophy" /> |
|||
==Pathophysiology== |
==Pathophysiology== |
||
Although there are nearly |
Although there are nearly 40 different types of leukodystrophy, many are lacking in formal and comprehensive research. Most of the research so far has been done on five types: (1) [[metachromatic leukodystrophy]] (MLD), (2) [[Krabbe disease]], (3) X-Linked [[adrenoleukodystrophy]] (ALD), (4) [[Canavan disease]], and (5) [[Alexander disease]]. Each type of leukodystrophy has a unique [[pathophysiology]], but all five of these in some way affect a subset of glial cells, therefore disrupting myelin production and maintenance, and usually involve a mutation involving genes that code for enzymes necessary for the [[catabolism]] of [[very long chain fatty acids]] (VLCFAs) that are toxic to the myelin-producing cells of the central nervous system.<ref name=VFCLAs>{{cite journal|last1=Sassa|first1=Takayuki|last2=Kihara|first2=Akio|title=Metabolism of Very Long-Chain Fatty Acids: Genes and Pathophysiology|journal=Biomolecules & Therapeutics|date=March 22, 2014|volume=22|issue=2|pages=83–92|doi=10.4062/biomolther.2014.017|pmc=3975470|pmid=24753812}}</ref> |
||
===Metachromatic leukodystrophy=== |
===Metachromatic leukodystrophy=== |
||
{{Main|Metachromatic leukodystrophy}} |
{{Main|Metachromatic leukodystrophy}} |
||
[[Metachromatic leukodystrophy]] is the result of genetic defects in the enzymes associated with the cellular compartment the [[lysosome]]. MLD is one of two leukodystophies that are also a [[lysosomal storage disorder]]. MLD is inherited in an [[autosomal recessive]] way and is the result of mutations in three different ARSA [[alleles]] that encode the enzyme [[arylsulfatase A]] (ASA or sometimes ARSA), also called [[sulfatide]] [[sulfatase]].<ref name="Metachromatic - ABSTRACT only">{{cite journal|last1=Barboura|first1=Ilhem|last2=Ferchichi|first2=Salima|last3=Dandana|first3=Azza|last4=Jaidane|first4=Zaineb|last5=Ben Khelifa|first5=Souhaira|last6=Chahed|first6=Hinda|last7=Ben Mansour|first7=Rachida|last8=Chebel|first8=Saber|last9=Maire|first9=Irene|last10=Miled|first10=Abdelhedi|title=Metachromatic leucodystrophy. Clinical, biological, and therapeutic aspects|journal=Annales de Biologie Clinique|date=2010|volume=68|issue=4|pages=385–91|pmid=20650733|doi=10.1684/abc.2010.0448}}</ref> ASA is responsible for the breakdown of sulfatides, [[sphingolipids]] present in neuronal membranes as well as in myelin. When there is a mutation in the gene that encodes ASA, |
[[Metachromatic leukodystrophy]] is the result of genetic defects in the enzymes associated with the cellular compartment called the [[lysosome]]. MLD is one of two leukodystophies that are also a [[lysosomal storage disorder]]. MLD is inherited in an [[autosomal recessive]] way and is the result of mutations in three different ARSA [[alleles]] that encode the enzyme [[arylsulfatase A]] (ASA or sometimes ARSA), also called [[sulfatide]] [[sulfatase]].<ref name="Metachromatic - ABSTRACT only">{{cite journal|last1=Barboura|first1=Ilhem|last2=Ferchichi|first2=Salima|last3=Dandana|first3=Azza|last4=Jaidane|first4=Zaineb|last5=Ben Khelifa|first5=Souhaira|last6=Chahed|first6=Hinda|last7=Ben Mansour|first7=Rachida|last8=Chebel|first8=Saber|last9=Maire|first9=Irene|last10=Miled|first10=Abdelhedi|title=Metachromatic leucodystrophy. Clinical, biological, and therapeutic aspects|journal=Annales de Biologie Clinique|date=2010|volume=68|issue=4|pages=385–91|pmid=20650733|doi=10.1684/abc.2010.0448}}</ref> ASA is responsible for the breakdown of sulfatides, [[sphingolipids]] present in neuronal membranes as well as in myelin. When there is a mutation in the gene that encodes ASA, it decreases ASA production, which subsequently leads to diminished degradation of sulfatides, thus causing them to accumulate.<ref name="Metachromatic - ABSTRACT only" /> This accumulation of sulfatides is toxic to oligodendrocytes, the myelin-producing cells of the CNS, effectively leading to a disturbance in myelin structure followed by [[demyelination]]. The pattern of inheritance of the three different alleles affects what type of MLD a person develops. Two [[null alleles]] are responsible for the infantile version, and do not allow for any production of ASA. A [[heterozygous]] individual (one null allele, one non-null allele) develops the juvenile form and has some production of ASA, while an individual with two mutated non-null alleles develops the adult form.<ref name="Metachromatic Leuko 2. - ABSTRACT ONLY">{{cite journal|last1=Gieselman|first1=V|last2=Krageloh-Mann|first2=I|title=Metachromatic Leukodystrophy - An Update|journal=Neuropediatrics|date=2010|volume=41|issue=1|pages=1–6|pmid=20571983|doi=10.1055/s-0030-1253412}}</ref> |
||
===Krabbe disease=== |
===Krabbe disease=== |
||
[[File:Globoid cell leukodystrophy PAS.jpg|thumb|Globoid cell leukodystrophy PAS - Multinucleated macrophages ("globoid cells") and loss of myelinated fibers in a |
[[File:Globoid cell leukodystrophy PAS.jpg|thumb|Globoid cell leukodystrophy PAS - Multinucleated macrophages ("globoid cells") and loss of myelinated fibers in a tissue sample of Krabbe's leukodystrophy]] |
||
{{Main|Krabbe disease}} |
{{Main|Krabbe disease}} |
||
Like MLD, [[Krabbe disease]] is another type of leukodystrophy with autosomal recessive inheritance that is the result of a [[lysosomal storage disorder]]. It is due to a deletion in exon 16 of the [[GALC |
Like MLD, [[Krabbe disease]] is another type of leukodystrophy with autosomal recessive inheritance that is the result of a [[lysosomal storage disorder]]. It is due to a deletion in exon 16 of the [[GALC (gene)|GALC gene]] that causes a [[frameshift mutation]] leading to a premature [[stop codon]]. The GALC gene, found on [[chromosome 14]] at position 31 (14q31), codes for the [[enzyme]] beta-galactocerebrosidase (GALC).<ref name="Szymanska - Krabbe">{{cite journal|last1=Szymanska|first1=Krystyna|last2=Lugowska|first2=Agnieszka|last3=Laure-Kamionowska|first3=Milena|last4=Gieruszczak-Bialek|first4=Dorota|last5=Musielak|first5=Malgorzata|last6=Eichler|first6=Sabrina|last7=Giese|first7=Anne-Katrin|last8=Rolfs|first8=Arndt|title=Diagnostic difficulties in Krabbe disesase: a report of two cases and review of literature|journal=Folia Neuropathol|date=2012|volume=50|issue=4|pages=346–356|pmid=23319190|doi=10.5114/fn.2012.32364|doi-access=free}}</ref> GALC is a lysosomal enzyme responsible for the catabolism of [[galactolipids]], especially the toxic lipid [[psychosine]], that are widely distributed throughout the brain. A deficiency in GALC thus causes a buildup of these [[fatty acids]], leading to an incursion by cells called "globoid [[macrophages]]" that destroy oligodendrocytes, thereby inhibiting any further myelin formation.<ref name="Globoid cells and arylsufatase - ABSTRACT ONLY">{{cite book|last1=Kohlschutter|first1=Alfried|chapter=Lysosomal leukodystrophies |title=Pediatric Neurology Part III|series=Handbook of Clinical Neurology|date=April 25, 2013|volume=113|issue=Pediatric Neurology Part III|pages=1611–1618|doi=10.1016/B978-0-444-59565-2.00029-0|pmid=23622382|isbn=9780444595652}}</ref> Given the presence of globoid macrophages clustered near [[white matter]], Krabbe disease often is called globoid cell leukodystrophy. |
||
Because of the presence of globoid cells clustered near [[white matter]], Krabbe disease often goes by the name globoid cell leukodystrophy. Furthermore, new research has shown that Krabbe disease and globoid cell leukodystrophy may be distinct disease entities due to the secretion of [[inflammatory mediators]] by [[natural killer cells]] in some cases.<ref name="Natural Killer Cells">{{cite journal|last1=Maghazachi|first1=Azzam A.|title=On the Role of Natural Killer Cells in Neurodegenerative Diseases|journal=Toxins (Basel)|date=February 5, 2013|volume=5|issue=2|pages=363–375|pmc=3640540|pmid=23430541|doi=10.3390/toxins5020363}}</ref> This research has shown that Natural Killer cells have receptors (TDAG8) for certain [[glycosphingolipids]] that build up in an individual with leukodystrophy, again due to insufficient GALC levels, and when bound, target the Natural Killer cells for destruction thereby preventing their [[cytotoxic]] effects. These sphingolipids have been identified as galactosyl sphingosine and glycosyl sphingosine and are not present in unaffected individuals.<ref name="Natural Killer Cells" /> |
|||
===Canavan disease=== |
===Canavan disease=== |
||
{{Main|Canavan disease}} |
{{Main|Canavan disease}} |
||
[[Canavan disease]] is a |
[[Canavan disease]] is a less-studied type of leukodystrophy that, like MLD and Krabbe disease, is also inherited in an autosomal recessive pattern. It is due to a mutation in [[ASPA (gene)|the ASPA gene]] that encodes [[aspartoacylase]], an enzyme needed to metabolize [[N-acetyl-L-aspartate]] (NAA). The mutation causes a deficiency of aspartoacyclase. NAA is involved in the formation of [[lipids]]; if it is not broken down by aspartoacylase, lipid levels in the brain increase, causing demyelination.<ref name="Canavan Disease - ULF">{{cite web|last1=United Leukodystrophy Foundation|title=Canavan Disease|url=http://ulf.org/canavan-disease|website=United Leukodystrophy Foundation|publisher=United Leukodystrophy Foundation, Inc.|access-date=March 30, 2015}}</ref> |
||
===X-linked adrenoleukodystrophy=== |
===X-linked adrenoleukodystrophy=== |
||
{{Main|Adrenoleukodystrophy}} |
{{Main|Adrenoleukodystrophy}} |
||
In X-linked adrenoleukodystrophy (X-ALD), a mutation occurs in the [[peroxisomal]] [[ATP-binding cassette]] ([[ABC transporter]]). This leads to cerebral inflammatory [[demyelination]] caused by |
In X-linked adrenoleukodystrophy (X-ALD), a mutation occurs in the [[peroxisomal]] [[ATP-binding cassette]] ([[ABC transporter]]). This leads to cerebral inflammatory [[demyelination]] caused by myelin destabilization.<ref name=berger>{{cite journal|last1=Berger|first1=J|last2=Forss-Petter|first2=S|last3=Eichler|first3=F.S.|title=Pathophysiology of X-Linked Adrenoleukodystrophy|journal=Biochimie|date=March 2014|volume=98|issue=100|pages=135–142|doi=10.1016/j.biochi.2013.11.023|pmid=24316281|pmc=3988840}}</ref> The inflammatory demyelination begins in the [[corpus callosum]] and slowly progresses outwards into both hemispheres. In X-ALD patients, abnormally high levels of VLCFA accumulate in various body tissues and fluids. This increased concentration then incorporates into various complex lipids where VLCFAs are not normally found.<ref name=berger /> This has been found to be directly involved in the cerebral inflammation of X-ALD. It is speculated that the accumulated and embedded VLCFA in the complex lipids could lead to the destabilization of the myelin sheath and eventually to demyelination.{{citation needed|date=September 2020}} |
||
===Alexander disease=== |
===Alexander disease=== |
||
{{Main|Alexander disease}} |
{{Main|Alexander disease}} |
||
[[Alexander disease]] is unique from the leukodystrophies mentioned above in that it is the result of [[spontaneous mutation]], that |
[[Alexander disease]] is unique from the leukodystrophies mentioned above, in that it is the result of [[spontaneous mutation]], meaning that it is not inherited. The mutation found in an affected individual is not found in either of his or her parents. Symptoms result from the accumulation of [[Glial fibrillary acidic protein]] (GFAP) as the result of a mutation in [[GFAP (gene)|the GFAP gene]], whose protein, rather than being found in association with lysosomes or peroxisomes, is an [[intermediate filament]] linked to the [[nuclear envelope]].<ref name="Alexander's Disease">{{cite journal|last1=Singh|first1=Navneet|last2=Bixby|first2=Catherine|last3=Etienne|first3=Denzil|last4=Tubbs|first4=R. Shane|last5=Loukas|first5=Marios|title=Alexander's disease: reassessment of a neonatal form|journal=Child's Nervous System|date=December 2012|volume=28|issue=12|pages=2029–2031|doi=10.1007/s00381-012-1868-8|pmid=22890470|s2cid=5851209}}</ref> Intermediate filaments are proteins responsible for the makeup of the cellular [[cytoskeleton]]; thus, this type of mutation causes abnormal structural development of a person's cells. [[Cytoskeleton|Cytoskeletal]] and [[protein transport|transporter molecule]] defects have been observed in the [[astrocytes]] of affected individuals. These astrocytes contain abnormally high levels of GFAP protein, affecting their development and function.<ref name=GFAP>{{cite journal|last1=Hol|first1=Elly M.|last2=Pekny|first2=Milos|title=Glial fibrillary acidic protein (GFAP) and the astrocyte intermediate filament system in diseases of the central nervous system|journal=Current Opinion in Cell Biology|date=February 2015|volume=32|issue=Cell Architecture|pages=121–130|doi=10.1016/j.ceb.2015.02.004|pmid=25726916}}</ref> |
||
==Diagnosis== |
==Diagnosis== |
||
The degeneration of [[white matter]], which |
The degeneration of [[white matter]], which reflects the degeneration of myelin, can be seen in a basic [[MRI]] and used to diagnose leukodystrophies of all types. T-1 and T-2 weighted [[fluid-attenuated inversion recovery]] (FLAIR) images are the most often used approach.<ref name="Diagnosis - MRI">{{cite journal|last1=Kohlschutter|first1=Alfried|last2=Eichler|first2=Florian|title=Childhood leukodystrophies: a clinical perspective|journal=Expert Review of Neurotherapeutics|date=October 2011|volume=11|issue=10|pages=1485–1496|doi=10.1586/ern.11.135|pmid=21955203|s2cid=27471268|url=https://zenodo.org/record/3440302}}{{Dead link|date=March 2022 |bot=InternetArchiveBot |fix-attempted=yes }}</ref> Electrophysiological and other kinds of laboratory testing can also be done. In particular, [[nerve conduction velocity]] is looked at to distinguish between leukodystrophy and other [[demyelinating diseases]], as well as to distinguish between individual leukodystrophies. For example, individuals with X-ALD have normal conduction velocities, while those with Krabbe disease or metachromatic leukodystrophy have abnormalities in their conduction velocities.<ref name="Diagnosis - MRI" /> Multigene sequencing panels for undifferentiated leukodystrophy are offered for rapid molecular diagnosis after genetic counselling.{{citation needed|date=September 2020}} |
||
===Types=== |
===Types=== |
||
Specific types of |
Specific types of leukodystrophy include the following with their respective [[ICD|ICD-10]] codes when available:{{citation needed|date=September 2020}} |
||
* (E71.3) [[Adrenomyeloneuropathy]] |
|||
* (E75.2) [[Alexander disease]] |
* (E75.2) [[Alexander disease]] |
||
| ⚫ | |||
| ⚫ | |||
| ⚫ | |||
| ⚫ | |||
| ⚫ | |||
* (E75.5) [[Cerebrotendineous xanthomatosis]] |
* (E75.5) [[Cerebrotendineous xanthomatosis]] |
||
* [[Hereditary CNS demyelinating disease]] |
|||
| ⚫ | |||
| ⚫ | |||
| ⚫ | |||
| ⚫ | |||
<!--This section's sources may be outdated, with the most recent one being from 2016. The entire section may need a rewrite, to reflect the current state of practice and understanding.--> |
|||
| ⚫ | |||
| ⚫ | |||
| ⚫ | |||
** (E71.3) [[Adrenoleukodystrophy]] |
|||
* (G60.1) [[Refsum disease]] |
|||
==Treatment== |
==Treatment== |
||
With many different types of |
With many different types of leukodystrophy, with many causes, treatment therapies will vary for each type. Studies and clinical trials are seeking to find therapies for each of the different leukodystrophies. [[Stem cell]] transplants and [[gene therapy]] appear to be the most promising in treating all leukodystrophies, providing they take place as early as possible, prior to extensive neurological damage. |
||
For hypomyelinating leukodystrophies, therapeutic research into cell-based therapies appears promising. [[Oligodendrocyte]] precursor cells and [[neural stem cells]] have been transplanted successfully and have shown to be healthy a year later. [[Fractional anisotropy |
For hypomyelinating leukodystrophies, therapeutic research into cell-based therapies appears promising. [[Oligodendrocyte]] precursor cells and [[neural stem cells]] have been transplanted successfully and have shown to be healthy a year later. [[diffusion tensor imaging|Fractional anisotropy and radial diffusivity maps]] showed possible myelination in the region of the transplant.<ref name=Pouwels>{{cite journal|last1=Pouwels|first1=P. J. W.|last2=Vanderver|first2=A.|last3=Bernard|first3=G.|last4=Wolf|first4=N.|last5=Dreha-Kulczewski|first5=S. W.|last6=Deoni|first6=S. C. L.|last7=Bertini|first7=E.|last8=Kohlschutter|first8=A.|last9=Richardson|first9=W.|last10=ffrench-Constant|first10=C.|last11=Kohler|first11=W.|last12=Barkovich|first12=A.|title=Hypomyelinating Leukodystrophies: Translational Research Progress and Prospects|journal=Ann. Neurol.|date=2014|doi=10.1002/ana.24194|volume=76|issue=1|pages=5–19|pmid=24916848|s2cid=19026052|url=https://www.pure.ed.ac.uk/ws/files/16949724/Hypomyelinating_leukodystrophies_Translational_research_progress_and_prospects.pdf|doi-access=free}}</ref>{{update after|2023|2|22}} [[Induced pluripotent stem cells]], oligodendrocyte precursor cells, gene correction, and transplantation to promote the maturation, survival, and myelination of [[oligodendrocytes]] seem to be the primary routes for possible treatments.<ref name="Pouwels"/>{{update after|2023|2|22}} |
||
For three types of leukodystrophies ([[X-linked adrenoleukodystrophy]] (X-ALD), [[metachromatic leukodystrophy]] (MLD) and [[Krabbe Disease]] (globoid cell leukodystrophy - GLD), gene therapy using autologous [[hematopoietic stem cells]] to transfer the disease gene with [[lentiviral]] [[Viral vector|vector]]s |
For three types of leukodystrophies ([[X-linked adrenoleukodystrophy]] (X-ALD), [[metachromatic leukodystrophy]] (MLD) and [[Krabbe Disease]] (globoid cell leukodystrophy - GLD), gene therapy using autologous [[hematopoietic stem cells]] to transfer the healthy copy of the disease-causing gene with [[lentiviral]] [[Viral vector|vector]]s has been shown to succeed and has been used in clinical trials for X-ALD and MLD.<ref name=Biffi>{{Cite journal |doi = 10.1093/hmg/ddr142|pmid = 21459776|title = Gene therapy for leukodystrophies|journal = Human Molecular Genetics|volume = 20|issue = R1|pages = R42–R53|year = 2011|last1 = Biffi|first1 = A.|last2 = Aubourg|first2 = P.|last3 = Cartier|first3 = N.|doi-access = free}}</ref><ref name="nhs-mld-2015">{{cite web |title=First baby receives life-saving gene therapy on NHS |url=https://www.england.nhs.uk/2023/02/first-baby-receives-life-saving-gene-therapy-on-nhs/ |website=NHS England |publisher=NHS |access-date=18 February 2023 |date=15 Feb 2023}}</ref> The progression of X-ALD has been shown to be disrupted with hematopoietic stem cell gene therapy, although the [[proximal cause]] of arrested [[demyelination]] and the quantity of stem cells needed are unclear.<ref name=Biffi /> While there continues to be an accumulation of [[very long chain fatty acids]] in the brain, this seems not to be the immediate causal factor behind the disease, as gene therapy does not correct the accumulation.<ref name=Biffi />{{update after|2023|2|22}} |
||
For those leukodystrophies that result from a deficiency of lysozyme enzymes, such as [[Krabbe disease]], enzyme replacement therapy seems hopeful. However, enzyme delivery proves difficult, because the [[ |
For those leukodystrophies that result from a deficiency of lysozyme enzymes, such as [[Krabbe disease]], enzyme replacement therapy seems hopeful. However, enzyme delivery proves difficult, because the [[blood–brain barrier]] severely limits what can pass into the central nervous system.<ref name=Biffi /> Current gene therapy research for metachromatic leukodystrophy has been reviewed with an emphasis on ''[[ex vivo]]'' transplantation of genetically modified hematopoietic stem cells.<ref>{{Cite journal|pmid = 27638601|year = 2016|last1 = Rosenberg|first1 = J. B.|last2 = Kaminsky|first2 = S. M.|last3 = Aubourg|first3 = P.|last4 = Crystal|first4 = R. G.|last5 = Sondhi|first5 = D.|title = Gene therapy for metachromatic leukodystrophy|journal = Journal of Neuroscience Research|volume = 94|issue = 11|pages = 1169–79|doi = 10.1002/jnr.23792|pmc = 5027970}}</ref>{{update after|2023|2|22}} |
||
==Epidemiology== |
==Epidemiology== |
||
[[File:2928 X-linked Recessive Inheritance-new.jpg|thumb|X-linked Recessive Inheritance]] |
[[File:2928 X-linked Recessive Inheritance-new.jpg|thumb|X-linked Recessive Inheritance]] |
||
Currently, no research has shown a higher prevalence of most |
Currently, no research has shown a higher prevalence of most leukodystrophy types in any one place around the world. There is, however, a higher prevalence of Canavan disease in the Jewish population. One in 40 individuals of [[Ashkenazi]] Jewish descent are carriers of Canavan disease.<ref name="Ashkenazi Jews/Canavan - ABSTRACT ONLY">{{cite journal|last1=Fiegenbaum|first1=Annette|last2=Moore|first2=Robert|last3=Clarke|first3=Joe|last4=Hewson|first4=Stacy|last5=Chityat|first5=David|last6=Ray|first6=Peter N.|last7=Stockley|first7=Tracy L.|title=Canavan disease: Carrier-frequency determination in the Ashkenazi Jewish population and development of a novel molecular diagnostic assay|journal=American Journal of Medical Genetics|date=January 15, 2004|volume=124A|issue=2|pages=142–7|doi=10.1002/ajmg.a.20334|pmid=14699612|s2cid=25981659}}</ref> This extrapolates to roughly 2.5%. Additionally, due to their autosomal recessive inheritance patterns, there is no significant difference found between males and females for most types of leukodystrophy, including but not limited to metachromatic leukodystrophy, Krabbe disease, Canavan disease, and Alexander disease. The one exception to this is any type of leukodystrophy carried on a [[sex chromosome]], such as X-linked adrenoleukodystrophy, which is carried on the X-chromosome. Because of the inheritance pattern of X-linked diseases, males are more often affected by this type of leukodystrophy, while female carriers are often symptomatic, though not as severely affected as males.<ref name="Genetics - Epidemiology - ABSTRACT">{{cite journal|last1=Lesca|first1=G|last2=Vanier|first2=MT|last3=Creisson|first3=E|last4=Bendelac|first4=N|last5=Hainque|first5=B|last6=Ollagnon-Roman|first6=E|last7=Aubourg|first7=P|title=X-linked adrenoleukodystrophy in a female proband: clinical presentation, biological diagnosis and family consequences|journal=Archives de Pédiatrie|date=August 2005|volume=12|issue=8|pages=1237–40|pmid=15878823|doi=10.1016/j.arcped.2005.03.050}}</ref> |
||
==Research== |
==Research== |
||
The [[National Institute of Neurological Disorders and Stroke]] (NINDS) supports research on genetic disorders, including the leukodystrophies.<ref name="NIH">{{cite web |title=Leukodystrophy Information Page |
The [[National Institute of Neurological Disorders and Stroke]] (NINDS, under the U.S. [[National Institutes of Health]]) supports research on genetic disorders, including the leukodystrophies.<ref name="NIH">{{cite web |title=Leukodystrophy Information Page |url=https://www.ninds.nih.gov/Disorders/All-Disorders/Leukodystrophy-Information-Page |website=www.ninds.nih.gov}}</ref> NINDS also supports researchers who are working with the Global Leukodystrophy Initiative Clinical Trials Network (GLIA-CTN) which promotes advances in the diagnosis and treatment of leukodystrophies.<ref name="glia">{{cite web |title=The Global Leukodystrophy Initiative |url=https://theglia.org |website=The Global Leukodystrophy Initiative}}</ref> |
||
The European Leukodystrophy Association also supports research into leukodystrophy. As of 2020, more than 387 research projects have been funded. Each year, ELA invites the international scientific community to submit research projects in the field of genetic leukodystrophies, the cerebral white matter in premature infants, and of myelin repair.<ref name="ELA">{{cite web |title=Accueil - |url=https://ela-asso.com |website=ela-asso.com}}</ref> |
The European Leukodystrophy Association also supports research into leukodystrophy. As of 2020, more than 387 research projects have been funded. Each year, ELA invites the international scientific community to submit research projects in the field of genetic leukodystrophies, the cerebral white matter in premature infants, and of myelin repair.<ref name="ELA">{{cite web |title=Accueil - |url=https://ela-asso.com |website=ela-asso.com}}</ref> |
||
| Line 107: | Line 107: | ||
The United Leukodystrophy Foundation (ULF), incorporated in 1982, is a non-profit, voluntary health organization dedicated to funding cutting-edge research and to providing patients and their families with disease information and medical referrals.<ref name="ULF">{{cite web |title=Research |url=https://ulf.org/research/ |website=United Leukodystrophy Foundation}}</ref> |
The United Leukodystrophy Foundation (ULF), incorporated in 1982, is a non-profit, voluntary health organization dedicated to funding cutting-edge research and to providing patients and their families with disease information and medical referrals.<ref name="ULF">{{cite web |title=Research |url=https://ulf.org/research/ |website=United Leukodystrophy Foundation}}</ref> |
||
Cure MLD is a global network of patient advocates and nonprofits dedicated to helping families impacted by [[metachromatic leukodystrophy]] (MLD).<ref name="cure">{{cite web |title=Home {{!}} |
Cure MLD is a global network of patient advocates and nonprofits dedicated to helping families impacted by [[metachromatic leukodystrophy]] (MLD).<ref name="cure">{{cite web |title=Home {{!}} Metachromatic leukodystrophy |url=https://curemld.com |website=curemld |language=en}}</ref> |
||
The ''MLD Foundation'' was co-founded by Dean and Teryn Suhr in 2001 after the diagnosis in 1995 of two of their daughters with MLD. MLD Foundation serves families and works with researchers, clinicians, regulators, payors, and policy-makers around the world on MLD, leukodystrophy, lysosomal, and rare disease issues.<ref name="mld">{{cite web |title=MLD Foundation |url=https://mldfoundation.org |website=mldfoundation.org}}</ref> |
The ''MLD Foundation'' was co-founded by Dean and Teryn Suhr in 2001 after the diagnosis in 1995 of two of their daughters with MLD. MLD Foundation serves families and works with researchers, clinicians, regulators, payors, and policy-makers around the world on MLD, leukodystrophy, lysosomal, and rare disease issues.<ref name="mld">{{cite web |title=MLD Foundation |url=https://mldfoundation.org |website=mldfoundation.org}}</ref> |
||
| Line 113: | Line 113: | ||
The ''Leukodystrophy Alliance'' works to promote awareness and quality of care for those with leukodystrophy.<ref name="LAO">{{cite web |title=leukodystrophyalliance.org - This website is for sale! - leukodystrophyalliance Resources and Information. |url=http://leukodystrophyalliance.org |website=leukodystrophyalliance.org}}</ref> |
The ''Leukodystrophy Alliance'' works to promote awareness and quality of care for those with leukodystrophy.<ref name="LAO">{{cite web |title=leukodystrophyalliance.org - This website is for sale! - leukodystrophyalliance Resources and Information. |url=http://leukodystrophyalliance.org |website=leukodystrophyalliance.org}}</ref> |
||
Jill Kelly and her husband, [[NFL]] [[quarterback]] [[Jim Kelly]], founded ''Hunter's Hope Foundation'' to fund research after their son Hunter ( |
Jill Kelly and her husband, [[NFL]] [[quarterback]] [[Jim Kelly]], founded ''Hunter's Hope Foundation'' to fund research after their son Hunter (1997–2005) was diagnosed with infantile Krabbe leukodystrophy.<ref name="HH">{{cite web |title=Please Help Leukodystrophy Children |url=https://www.classy.org/give/114114/#!/donation/checkout |website=www.classy.org}}</ref> |
||
Matthew and Michael Clark of [[Kingston upon Hull|Hull]], UK |
Matthew and Michael Clark of [[Kingston upon Hull|Hull]], UK had the condition. Both died, in 2013 and 2016 respectively.{{citation needed|date=April 2021}} Their story was the subject of the Channel 4 documentary ''The Curious Case of the Clark Brothers''.<ref>{{cite web |title=The Curious Case of the Clark Brothers - Channel 4 |url=http://www.channel4.com/programmes/the-curious-case-of-the-clark-brothers |publisher=[[Channel 4]] |access-date=26 November 2012 |date=20 November 2012|archive-url=https://web.archive.org/web/20121120022319/https://www.channel4.com/programmes/the-curious-case-of-the-clark-brothers|archive-date=20 November 2012|url-status=dead}}</ref><ref>{{cite news |title=The curious case of the boys who live backwards |url=https://www.independent.co.uk/life-style/health-and-families/health-news/curious-case-boys-who-live-backwards-8348395.html |archive-url=https://ghostarchive.org/archive/20220614/https://www.independent.co.uk/life-style/health-and-families/health-news/curious-case-boys-who-live-backwards-8348395.html |archive-date=2022-06-14 |url-access=subscription |url-status=live |access-date=2 April 2021 |work=[[The Independent]] |date=24 November 2012 |language=en}}</ref> |
||
[[Augusto, Michaela, and Lorenzo Odone|Augusto and Michaela Odone]] founded [[The Myelin Project]] after their son, [[Augusto, Michaela, and Lorenzo Odone|Lorenzo]] was diagnosed with Adrenoleukodystrophy (ALD). The 1992 film, ''[[Lorenzo's Oil]]'' is a true story about a boy |
[[Augusto, Michaela, and Lorenzo Odone|Augusto and Michaela Odone]] founded [[The Myelin Project]] after their son, [[Augusto, Michaela, and Lorenzo Odone|Lorenzo]] was diagnosed with Adrenoleukodystrophy (ALD). The 1992 film, ''[[Lorenzo's Oil]]'' is a true story about a boy with Adrenoleukodystrophy (ALD).{{citation needed|date=December 2020}} |
||
==See also== |
==See also== |
||
Revision as of 19:41, 13 April 2024
| Leukodystrophy | |
|---|---|
 | |
| T2 weighted axial scan of a human brain at the level of the caudate heads demonstrates marked loss of posterior white matter, with reduced volume and increased signal intensity. The anterior white matter is spared. Features are consistent with X-linked adrenoleukodystrophy. | |
| Specialty | Neurology |
Leukodystrophies are a group of, usually, inherited disorders, characterized by degeneration of the white matter in the brain.[1] The word leukodystrophy comes from the Greek roots leuko, "white", dys, "abnormal" and troph, "growth". The leukodystrophies are caused by imperfect growth or development of the glial cells which produce the myelin sheath, the fatty insulating covering around nerve fibers.[2] Leukodystrophies may be classified as hypomyelinating or demyelinating diseases, respectively, depending on whether the damage is present before birth or occurs after. While all leukodystrophies are the result of genetic mutations,[3] other demyelinating disorders have an autoimmune, infectious, or metabolic etiology.[4]
When damage occurs to white matter, subsequent immune responses can lead to inflammation in the central nervous system (CNS), along with the loss of myelin. The degeneration of white matter can be seen in an MRI scan and is used to diagnose leukodystrophy. Leukodystrophy is characterized by specific symptoms, including decreased motor function, muscle rigidity, and eventual degeneration of sight and hearing. While the disease is fatal, the age of onset is a key factor, as infants have a typical life expectancy of 2–8 years, while adults typically live more than a decade after onset. Treatment options are limited, although hematopoietic stem cell transplantations using bone marrow or cord blood seem to help in certain leukodystrophy types, while further research is being done.
The combined incidence of the leukodystrophies is estimated at 1 in 7,600.[5] The majority of types involve the inheritance of an X-linked recessive, or X-linked dominant trait, while others, although involving a defective gene, are the result of spontaneous mutation rather than genetic inheritance.
Symptoms and signs
Some specific symptoms vary from one type of leukodystrophy to the next, but the vast majority of symptoms are shared as the causes for the disease generally have the same effects. Symptoms are dependent on the age of onset, which is predominantly in infancy and early childhood, although the exact time of onset may be difficult to determine. Hyperirritability and hypersensitivity to the environment are common, as well as some tell-tale physical signs including muscle rigidity and a backwards-bent head.[6] Botox therapy is often used to treat patients with spasticity.[7] Juvenile and adult onsets display similar symptoms including a decrease or loss in hearing and vision. While children do experience optic and auditory degeneration, the course of the disease is usually too rapid, causing death relatively quickly, whereas adults may live with these conditions for many years. In children, spastic activity often precedes progressive ataxia and rapid cognitive deterioration which has been described as mental retardation.[8] Epilepsy is commonplace for patients of all ages.[9] More progressed patients show weakness in deglutition, leading to spastic coughing fits due to inhaled saliva. Classic symptomatic progression of juvenile X-linked adrenoleukodystrophy is shown in the 1992 film, Lorenzo's Oil.[10]
Course and timetable are dependent on the age of onset with infants showing a lifespan of 2–8 years, juveniles 2–10 years and adults typically 10+ years. Adults typically see an extended period of stability followed by a decline to a vegetative state and death.[6] While treatments do exist, most are in the experimental phase and can only promise a halt in the progression of symptoms, although some gene therapies have shown some symptomatic improvement.[11] The debilitating course of the disease has led to numerous philosophical and ethical arguments over experimental clinical trials, patients' rights and physician-assisted suicide.[12]
Causes
While the more specific underlying causes of leukodystrophy are dependent upon the type, there are common pathophysiological patterns that can be seen amongst all types. First and foremost, leukodystrophy is a neurodegenerative disease that is always the result of both impairment and maintenance of myelin sheaths surrounding neuronal axons in the central nervous system as the result of a genetic mutation.[13] Myelin is a fatty white substance that acts as an electrical insulator and coats axons in order to speed up impulses (i.e., action potentials) traveling down the axon. Thus, the natural result of a loss of this substance is decreased efficiency in impulse propagation. As myelin is produced by oligodendrocytes (a type of glial cell) in the central nervous system, an easy place to look for the cause is a mutation or malfunctioning of these cells and in other glial cells.[citation needed]
Genetic influence

Inherited forms of leukodystrophy are usually the result of an autosomal recessive inheritance pattern, although dominant inheritance patterns are not unheard of, as in the case of adult-onset leukodystrophy.[14] This means that the affected allele is carried on an autosomal, or non-sex, chromosome and is masked by the dominant, unaffected phenotype. In other words, for an individual to inherit the leukodystrophy phenotype, he or she must carry two of the recessive, mutant alleles. Krabbe disease and metachromatic leukodystrophy (MLD) are two of such type. MLD is found on human chromosome 22 at position q13.31.[15] Another type of inherited leukodystrophy is X-linked adrenoleukodystrophy (X-ALD). As its name implies, this type of leukodystrophy is the result of a mutation found on the X-chromosome. It is also carried in a recessive pattern. The X chromosome is a sex chromosome, and since women have two "chances" of acquiring a normal X chromosome (one maternal x, one paternal x), and males only one chance (one maternal x), this disease is more likely to be seen in males than in females. The mutation resulting in adult-onset leukodystrophy is mapped at 5q23.[14]
Pathophysiology
Although there are nearly 40 different types of leukodystrophy, many are lacking in formal and comprehensive research. Most of the research so far has been done on five types: (1) metachromatic leukodystrophy (MLD), (2) Krabbe disease, (3) X-Linked adrenoleukodystrophy (ALD), (4) Canavan disease, and (5) Alexander disease. Each type of leukodystrophy has a unique pathophysiology, but all five of these in some way affect a subset of glial cells, therefore disrupting myelin production and maintenance, and usually involve a mutation involving genes that code for enzymes necessary for the catabolism of very long chain fatty acids (VLCFAs) that are toxic to the myelin-producing cells of the central nervous system.[16]
Metachromatic leukodystrophy
Metachromatic leukodystrophy is the result of genetic defects in the enzymes associated with the cellular compartment called the lysosome. MLD is one of two leukodystophies that are also a lysosomal storage disorder. MLD is inherited in an autosomal recessive way and is the result of mutations in three different ARSA alleles that encode the enzyme arylsulfatase A (ASA or sometimes ARSA), also called sulfatide sulfatase.[17] ASA is responsible for the breakdown of sulfatides, sphingolipids present in neuronal membranes as well as in myelin. When there is a mutation in the gene that encodes ASA, it decreases ASA production, which subsequently leads to diminished degradation of sulfatides, thus causing them to accumulate.[17] This accumulation of sulfatides is toxic to oligodendrocytes, the myelin-producing cells of the CNS, effectively leading to a disturbance in myelin structure followed by demyelination. The pattern of inheritance of the three different alleles affects what type of MLD a person develops. Two null alleles are responsible for the infantile version, and do not allow for any production of ASA. A heterozygous individual (one null allele, one non-null allele) develops the juvenile form and has some production of ASA, while an individual with two mutated non-null alleles develops the adult form.[18]
Krabbe disease
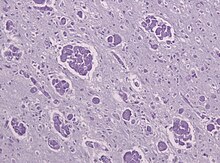
Like MLD, Krabbe disease is another type of leukodystrophy with autosomal recessive inheritance that is the result of a lysosomal storage disorder. It is due to a deletion in exon 16 of the GALC gene that causes a frameshift mutation leading to a premature stop codon. The GALC gene, found on chromosome 14 at position 31 (14q31), codes for the enzyme beta-galactocerebrosidase (GALC).[19] GALC is a lysosomal enzyme responsible for the catabolism of galactolipids, especially the toxic lipid psychosine, that are widely distributed throughout the brain. A deficiency in GALC thus causes a buildup of these fatty acids, leading to an incursion by cells called "globoid macrophages" that destroy oligodendrocytes, thereby inhibiting any further myelin formation.[20] Given the presence of globoid macrophages clustered near white matter, Krabbe disease often is called globoid cell leukodystrophy.
Canavan disease
Canavan disease is a less-studied type of leukodystrophy that, like MLD and Krabbe disease, is also inherited in an autosomal recessive pattern. It is due to a mutation in the ASPA gene that encodes aspartoacylase, an enzyme needed to metabolize N-acetyl-L-aspartate (NAA). The mutation causes a deficiency of aspartoacyclase. NAA is involved in the formation of lipids; if it is not broken down by aspartoacylase, lipid levels in the brain increase, causing demyelination.[21]
X-linked adrenoleukodystrophy
In X-linked adrenoleukodystrophy (X-ALD), a mutation occurs in the peroxisomal ATP-binding cassette (ABC transporter). This leads to cerebral inflammatory demyelination caused by myelin destabilization.[22] The inflammatory demyelination begins in the corpus callosum and slowly progresses outwards into both hemispheres. In X-ALD patients, abnormally high levels of VLCFA accumulate in various body tissues and fluids. This increased concentration then incorporates into various complex lipids where VLCFAs are not normally found.[22] This has been found to be directly involved in the cerebral inflammation of X-ALD. It is speculated that the accumulated and embedded VLCFA in the complex lipids could lead to the destabilization of the myelin sheath and eventually to demyelination.[citation needed]
Alexander disease
Alexander disease is unique from the leukodystrophies mentioned above, in that it is the result of spontaneous mutation, meaning that it is not inherited. The mutation found in an affected individual is not found in either of his or her parents. Symptoms result from the accumulation of Glial fibrillary acidic protein (GFAP) as the result of a mutation in the GFAP gene, whose protein, rather than being found in association with lysosomes or peroxisomes, is an intermediate filament linked to the nuclear envelope.[23] Intermediate filaments are proteins responsible for the makeup of the cellular cytoskeleton; thus, this type of mutation causes abnormal structural development of a person's cells. Cytoskeletal and transporter molecule defects have been observed in the astrocytes of affected individuals. These astrocytes contain abnormally high levels of GFAP protein, affecting their development and function.[24]
Diagnosis
The degeneration of white matter, which reflects the degeneration of myelin, can be seen in a basic MRI and used to diagnose leukodystrophies of all types. T-1 and T-2 weighted fluid-attenuated inversion recovery (FLAIR) images are the most often used approach.[25] Electrophysiological and other kinds of laboratory testing can also be done. In particular, nerve conduction velocity is looked at to distinguish between leukodystrophy and other demyelinating diseases, as well as to distinguish between individual leukodystrophies. For example, individuals with X-ALD have normal conduction velocities, while those with Krabbe disease or metachromatic leukodystrophy have abnormalities in their conduction velocities.[25] Multigene sequencing panels for undifferentiated leukodystrophy are offered for rapid molecular diagnosis after genetic counselling.[citation needed]
Types
Specific types of leukodystrophy include the following with their respective ICD-10 codes when available:[citation needed]
- (E75.2) Alexander disease
- (E75.2) Canavan disease
- (E75.2) Hypomyelinating leukodystrophy type 7 (4H syndrome)
- (E75.2) Krabbe disease
- (E75.2) Metachromatic leukodystrophy
- (E75.2) Pelizaeus–Merzbacher disease
- (E75.5) Cerebrotendineous xanthomatosis
Treatment
With many different types of leukodystrophy, with many causes, treatment therapies will vary for each type. Studies and clinical trials are seeking to find therapies for each of the different leukodystrophies. Stem cell transplants and gene therapy appear to be the most promising in treating all leukodystrophies, providing they take place as early as possible, prior to extensive neurological damage.
For hypomyelinating leukodystrophies, therapeutic research into cell-based therapies appears promising. Oligodendrocyte precursor cells and neural stem cells have been transplanted successfully and have shown to be healthy a year later. Fractional anisotropy and radial diffusivity maps showed possible myelination in the region of the transplant.[26][needs update] Induced pluripotent stem cells, oligodendrocyte precursor cells, gene correction, and transplantation to promote the maturation, survival, and myelination of oligodendrocytes seem to be the primary routes for possible treatments.[26][needs update]
For three types of leukodystrophies (X-linked adrenoleukodystrophy (X-ALD), metachromatic leukodystrophy (MLD) and Krabbe Disease (globoid cell leukodystrophy - GLD), gene therapy using autologous hematopoietic stem cells to transfer the healthy copy of the disease-causing gene with lentiviral vectors has been shown to succeed and has been used in clinical trials for X-ALD and MLD.[11][27] The progression of X-ALD has been shown to be disrupted with hematopoietic stem cell gene therapy, although the proximal cause of arrested demyelination and the quantity of stem cells needed are unclear.[11] While there continues to be an accumulation of very long chain fatty acids in the brain, this seems not to be the immediate causal factor behind the disease, as gene therapy does not correct the accumulation.[11][needs update]
For those leukodystrophies that result from a deficiency of lysozyme enzymes, such as Krabbe disease, enzyme replacement therapy seems hopeful. However, enzyme delivery proves difficult, because the blood–brain barrier severely limits what can pass into the central nervous system.[11] Current gene therapy research for metachromatic leukodystrophy has been reviewed with an emphasis on ex vivo transplantation of genetically modified hematopoietic stem cells.[28][needs update]
Epidemiology

Currently, no research has shown a higher prevalence of most leukodystrophy types in any one place around the world. There is, however, a higher prevalence of Canavan disease in the Jewish population. One in 40 individuals of Ashkenazi Jewish descent are carriers of Canavan disease.[29] This extrapolates to roughly 2.5%. Additionally, due to their autosomal recessive inheritance patterns, there is no significant difference found between males and females for most types of leukodystrophy, including but not limited to metachromatic leukodystrophy, Krabbe disease, Canavan disease, and Alexander disease. The one exception to this is any type of leukodystrophy carried on a sex chromosome, such as X-linked adrenoleukodystrophy, which is carried on the X-chromosome. Because of the inheritance pattern of X-linked diseases, males are more often affected by this type of leukodystrophy, while female carriers are often symptomatic, though not as severely affected as males.[30]
Forschung
The National Institute of Neurological Disorders and Stroke (NINDS, under the U.S. National Institutes of Health) supports research on genetic disorders, including the leukodystrophies.[31] NINDS also supports researchers who are working with the Global Leukodystrophy Initiative Clinical Trials Network (GLIA-CTN) which promotes advances in the diagnosis and treatment of leukodystrophies.[32]
The European Leukodystrophy Association also supports research into leukodystrophy. As of 2020, more than 387 research projects have been funded. Each year, ELA invites the international scientific community to submit research projects in the field of genetic leukodystrophies, the cerebral white matter in premature infants, and of myelin repair.[33]
Society
The United Leukodystrophy Foundation (ULF), incorporated in 1982, is a non-profit, voluntary health organization dedicated to funding cutting-edge research and to providing patients and their families with disease information and medical referrals.[34]
Cure MLD is a global network of patient advocates and nonprofits dedicated to helping families impacted by metachromatic leukodystrophy (MLD).[35]
The MLD Foundation was co-founded by Dean and Teryn Suhr in 2001 after the diagnosis in 1995 of two of their daughters with MLD. MLD Foundation serves families and works with researchers, clinicians, regulators, payors, and policy-makers around the world on MLD, leukodystrophy, lysosomal, and rare disease issues.[36]
The Leukodystrophy Alliance works to promote awareness and quality of care for those with leukodystrophy.[37]
Jill Kelly and her husband, NFL quarterback Jim Kelly, founded Hunter's Hope Foundation to fund research after their son Hunter (1997–2005) was diagnosed with infantile Krabbe leukodystrophy.[38]
Matthew and Michael Clark of Hull, UK had the condition. Both died, in 2013 and 2016 respectively.[citation needed] Their story was the subject of the Channel 4 documentary The Curious Case of the Clark Brothers.[39][40]
Augusto and Michaela Odone founded The Myelin Project after their son, Lorenzo was diagnosed with Adrenoleukodystrophy (ALD). The 1992 film, Lorenzo's Oil is a true story about a boy with Adrenoleukodystrophy (ALD).[citation needed]
See also
References
- ^ Sachdev, Perminder S.; Keshavan, Matcheri S. (2010-03-15). Secondary Schizophrenia. Cambridge University Press. pp. 241–. ISBN 978-0-521-85697-3. Retrieved 15 August 2011.
- ^
 One or more of the preceding sentences incorporates text from this source, which is in the public domain: "Leukodystrophy Information Page". National Institute of Neurological Disorders and Stroke. 25 May 2017. Retrieved 18 March 2018.
One or more of the preceding sentences incorporates text from this source, which is in the public domain: "Leukodystrophy Information Page". National Institute of Neurological Disorders and Stroke. 25 May 2017. Retrieved 18 March 2018.
- ^ "Leukodystrophy". www.ninds.nih.gov. Retrieved 2024-04-13.
- ^ Coggan, Jay S.; Bittner, Stefan; Stiefel, Klaus M.; Meuth, Sven G.; Prescott, Steven A. (September 2015). "Physiological Dynamics in Demyelinating Diseases: Unraveling Complex Relationships through Computer Modeling". International Journal of Molecular Sciences. 16 (9): 21215–21236. doi:10.3390/ijms160921215. ISSN 1422-0067. PMC 4613250. PMID 26370960.
- ^ Bonkowsky, Joshua (Aug 24, 2010). "The burden of inherited leukodystrophies in children". Neurology. 75 (8): 718–725. doi:10.1212/WNL.0b013e3181eee46b. PMC 2931652. PMID 20660364.
- ^ a b Graziano, AC; Cardile, V (26 September 2014). "History, genetic, and recent advances on Krabbe disease". Gene. 555 (1): 2–13. doi:10.1016/j.gene.2014.09.046. PMID 25260228.
- ^ Rosebush, P. I. (2003). "Tardive dystonia and its treatment". Journal of Psychiatry and Neuroscience. 28 (3): 240. PMC 161748.
- ^ Liu, Y; Zou, L; Meng, Y; Zhang, Y; Shi, X; Ju, J; Yang, G; Hu, L; Chen, X (June 2014). "[A family with two children diagnosed with aspartylglucosaminuria-case report and literature review]". Zhonghua Er Za Zhi. 52 (6): 455–9. PMID 25190167.
- ^ Turon-Vinas, E; Pineda, M; Cusi, V; Lopez-Laso, E; Del Pozo, RL; Gutierez-Solana, LG; Moreno, DC; Sierra-Corcoles, C; Olabarrieta-Hoyos, N; Madruga-Garrido, M; Aguirre-Rodriguez, J; Gonzalez-Alvarez, V; O'Callaghan, M; Muchart, J; Armstrong-Moron, J (13 July 2014). "Vanishing white matter disease in a Spanish population". J Cent Nerv Syst Dis. 6: 59–68. doi:10.4137/JCNSD.S13540. PMC 4116383. PMID 25089094.
- ^ Rubin, Rita (March 13, 2016). "Forbes.com: Lorenzo's Oil Could Not Cure Lorenzo, But Newborn Screening Is Expected To Save Others From His Fate". Forbes.com. Retrieved July 31, 2018..
- ^ a b c d e Biffi, A.; Aubourg, P.; Cartier, N. (2011). "Gene therapy for leukodystrophies". Human Molecular Genetics. 20 (R1): R42–R53. doi:10.1093/hmg/ddr142. PMID 21459776.
- ^ Duchange, N; Darguy, S; d'Audiffret, D; Callies, I; Lapointe, AS; Loeve, B; Boespflug-Tanguy, O; Moutel, G (18 September 2014). "Ethical management in the constitution of a European database for leukodystrophies rare diseases". Eur J Paediatr Neurol. 18 (5): 597–603. doi:10.1016/j.ejpn.2014.04.002. PMID 24786336. S2CID 31385905.
- ^ Yang, Edward; Prabhu, Sanjay P. (March 5, 2014). "Imaging manifestations of the leukodystrophies, inherited disorders of white matter". Radiologic Clinics of North America. 52 (2): 279–319. doi:10.1016/j.rcl.2013.11.008. PMID 24582341.
- ^ a b Lin, Shu-Ting; Ptacek, Louis J.; Fu, Ying-Hui (January 26, 2011). "Adult-Onset Autosomal Dominant Leukodystrophy: Linking Nuclear Envelope to Myelin". The Journal of Neuroscience. 31 (4): 1163–1166. doi:10.1523/jneurosci.5994-10.2011. PMC 3078713. PMID 21273400.
- ^ Coulter-Mackie, MB; Rip, J; Ludman, MD; Beis, J; Cole, DEC (October 1995). "Metachromatic leucodystrophy (MLD) in a patient with a constitutional ring chromosome 22". Journal of Medical Genetics. 32 (10): 787–91. doi:10.1136/jmg.32.10.787. PMC 1051701. PMID 8558556.
- ^ Sassa, Takayuki; Kihara, Akio (March 22, 2014). "Metabolism of Very Long-Chain Fatty Acids: Genes and Pathophysiology". Biomolecules & Therapeutics. 22 (2): 83–92. doi:10.4062/biomolther.2014.017. PMC 3975470. PMID 24753812.
- ^ a b Barboura, Ilhem; Ferchichi, Salima; Dandana, Azza; Jaidane, Zaineb; Ben Khelifa, Souhaira; Chahed, Hinda; Ben Mansour, Rachida; Chebel, Saber; Maire, Irene; Miled, Abdelhedi (2010). "Metachromatic leucodystrophy. Clinical, biological, and therapeutic aspects". Annales de Biologie Clinique. 68 (4): 385–91. doi:10.1684/abc.2010.0448. PMID 20650733.
- ^ Gieselman, V; Krageloh-Mann, I (2010). "Metachromatic Leukodystrophy - An Update". Neuropediatrics. 41 (1): 1–6. doi:10.1055/s-0030-1253412. PMID 20571983.
- ^ Szymanska, Krystyna; Lugowska, Agnieszka; Laure-Kamionowska, Milena; Gieruszczak-Bialek, Dorota; Musielak, Malgorzata; Eichler, Sabrina; Giese, Anne-Katrin; Rolfs, Arndt (2012). "Diagnostic difficulties in Krabbe disesase: a report of two cases and review of literature". Folia Neuropathol. 50 (4): 346–356. doi:10.5114/fn.2012.32364. PMID 23319190.
- ^ Kohlschutter, Alfried (April 25, 2013). "Lysosomal leukodystrophies". Pediatric Neurology Part III. Handbook of Clinical Neurology. Vol. 113. pp. 1611–1618. doi:10.1016/B978-0-444-59565-2.00029-0. ISBN 9780444595652. PMID 23622382.
- ^ a b Berger, J; Forss-Petter, S; Eichler, F.S. (March 2014). "Pathophysiology of X-Linked Adrenoleukodystrophy". Biochimie. 98 (100): 135–142. doi:10.1016/j.biochi.2013.11.023. PMC 3988840. PMID 24316281.
- ^ Singh, Navneet; Bixby, Catherine; Etienne, Denzil; Tubbs, R. Shane; Loukas, Marios (December 2012). "Alexander's disease: reassessment of a neonatal form". Child's Nervous System. 28 (12): 2029–2031. doi:10.1007/s00381-012-1868-8. PMID 22890470. S2CID 5851209.
- ^ Hol, Elly M.; Pekny, Milos (February 2015). "Glial fibrillary acidic protein (GFAP) and the astrocyte intermediate filament system in diseases of the central nervous system". Current Opinion in Cell Biology. 32 (Cell Architecture): 121–130. doi:10.1016/j.ceb.2015.02.004. PMID 25726916.
- ^ a b Kohlschutter, Alfried; Eichler, Florian (October 2011). "Childhood leukodystrophies: a clinical perspective". Expert Review of Neurotherapeutics. 11 (10): 1485–1496. doi:10.1586/ern.11.135. PMID 21955203. S2CID 27471268.[permanent dead link]
- ^ a b Pouwels, P. J. W.; Vanderver, A.; Bernard, G.; Wolf, N.; Dreha-Kulczewski, S. W.; Deoni, S. C. L.; Bertini, E.; Kohlschutter, A.; Richardson, W.; ffrench-Constant, C.; Kohler, W.; Barkovich, A. (2014). "Hypomyelinating Leukodystrophies: Translational Research Progress and Prospects" (PDF). Ann. Neurol. 76 (1): 5–19. doi:10.1002/ana.24194. PMID 24916848. S2CID 19026052.
- ^ "First baby receives life-saving gene therapy on NHS". NHS England. NHS. 15 Feb 2023. Retrieved 18 February 2023.
- ^ Rosenberg, J. B.; Kaminsky, S. M.; Aubourg, P.; Crystal, R. G.; Sondhi, D. (2016). "Gene therapy for metachromatic leukodystrophy". Journal of Neuroscience Research. 94 (11): 1169–79. doi:10.1002/jnr.23792. PMC 5027970. PMID 27638601.
- ^ Lesca, G; Vanier, MT; Creisson, E; Bendelac, N; Hainque, B; Ollagnon-Roman, E; Aubourg, P (August 2005). "X-linked adrenoleukodystrophy in a female proband: clinical presentation, biological diagnosis and family consequences". Archives de Pédiatrie. 12 (8): 1237–40. doi:10.1016/j.arcped.2005.03.050. PMID 15878823.
- ^ "Leukodystrophy Information Page". www.ninds.nih.gov.
- ^ "The Global Leukodystrophy Initiative". The Global Leukodystrophy Initiative.
- ^ "Accueil -". ela-asso.com.
- ^ "Research". United Leukodystrophy Foundation.
- ^ "Home | Metachromatic leukodystrophy". curemld.
- ^ "MLD Foundation". mldfoundation.org.
- ^ "leukodystrophyalliance.org - This website is for sale! - leukodystrophyalliance Resources and Information". leukodystrophyalliance.org.
{{cite web}}: Cite uses generic title (help) - ^ "Please Help Leukodystrophy Children". www.classy.org.
- ^ "The Curious Case of the Clark Brothers - Channel 4". Channel 4. 20 November 2012. Archived from the original on 20 November 2012. Retrieved 26 November 2012.
- ^ "The curious case of the boys who live backwards". The Independent. 24 November 2012. Archived from the original on 2022-06-14. Retrieved 2 April 2021.
