Birnessite: Difference between revisions
m Bot: galleries syntax and minor changes |
Randy Kryn (talk | contribs) →Properties and applications: uppercase per direct link (Baltic Sea) |
||
| (21 intermediate revisions by 7 users not shown) | |||
| Line 8: | Line 8: | ||
| imagesize = 260px |
| imagesize = 260px |
||
| caption = |
| caption = |
||
| formula = MnO<sub>2</sub> |
| formula = MnO<sub>2</sub>·''n''H<sub>2</sub>O<br />δ-{{chem2|MnO2}} |
||
| IMAsymbol=Bir<ref>{{Cite journal|last=Warr|first=L.N.|date=2021|title=IMA–CNMNC approved mineral symbols |
| IMAsymbol=Bir<ref>{{Cite journal|last=Warr|first=L.N.|date=2021|title=IMA–CNMNC approved mineral symbols|journal=Mineralogical Magazine|volume=85|issue=3|pages=291–320|doi=10.1180/mgm.2021.43|bibcode=2021MinM...85..291W|s2cid=235729616|doi-access=free}}</ref> |
||
| molweight = |
| molweight = |
||
| system = [[Triclinic]] or [[Hexagonal]] |
| system = [[Triclinic]] or [[Hexagonal]] |
||
| Line 22: | Line 22: | ||
| polish = |
| polish = |
||
| refractive = nω = 1.730 nε = 1.690 |
| refractive = nω = 1.730 nε = 1.690 |
||
| opticalprop = Uniaxial ( |
| opticalprop = Uniaxial (−) |
||
| birefringence = δ = 0.040 |
| birefringence = δ = 0.040 |
||
| dispersion = |
| dispersion = |
||
| Line 39: | Line 39: | ||
}} |
}} |
||
'''Birnessite''' (nominally MnO<sub>2</sub> |
'''Birnessite''' (nominally MnO<sub>2</sub>·''n''H<sub>2</sub>O), also known as δ-{{chem2|MnO2}}, is a hydrous manganese dioxide [[mineral]] with a chemical formula of Na<sub>0.7</sub>Ca<sub>0.3</sub>Mn<sub>7</sub>O<sub>14</sub>·2.8H<sub>2</sub>O.<ref>{{Cite journal|last1=Jones|first1=L. H. P.|display-authors=1|last2=Milne|first2=Angela A.|date=1956|title=Birnessite, a new manganese oxide mineral from Aberdeenshire, Scotland|url=http://dx.doi.org/10.1180/minmag.1956.031.235.01|journal=Mineralogical Magazine and Journal of the Mineralogical Society|volume=31|issue=235|pages=283–288|doi=10.1180/minmag.1956.031.235.01|bibcode=1956MinM...31..283J}}</ref> It is the main [[manganese]] mineral species at the Earth's surface, and commonly occurs as fine-grained, poorly crystallized aggregates in [[soil]]s, [[sediment]]s, grain and rock coatings (e.g., [[desert varnish]]), and marine [[ferromanganese nodules]] and crusts.<ref>{{Cite journal|last1=McKeown|first1=D. A.|display-authors=1|last2=Post|first2=Jeffrey E.|date=2001-05-01|title=Characterization of manganese oxide mineralogy in rock varnish and dendrites using X-ray absorption spectroscopy|url=https://www.degruyter.com/document/doi/10.2138/am-2001-5-611/html|journal=American Mineralogist|language=en|volume=86|issue=5–6|pages=701–713|doi=10.2138/am-2001-5-611|bibcode=2001AmMin..86..701M|s2cid=19647389}}</ref><ref>{{Cite journal|last1=[[Alain Manceau|Manceau]]|first1=A|display-authors=1|last2=Tamura|first2=Nobumichi|last3=Celestre|first3=Richard S.|last4=MacDowell|first4=Alastair A.|last5=Geoffroy|first5=Nicolas|last6=Sposito|first6=Garrison|last7=Padmore|first7=Howard A.|date=2003|title=Molecular-Scale Speciation of Zn and Ni in Soil Ferromanganese Nodules from Loess Soils of the Mississippi Basin|url=https://pubs.acs.org/doi/10.1021/es025748r|journal=Environmental Science & Technology|language=en|volume=37|issue=1|pages=75–80|doi=10.1021/es025748r|pmid=12542293|bibcode=2003EnST...37...75M}}</ref><ref name=":0">{{Cite journal|last1=Isaure|first1=M-P|display-authors=1|last2=[[Alain Manceau|Manceau]]|first2=A|last3=Geoffroy|first3=Nicolas|last4=Laboudigue|first4=Agnès|last5=Tamura|first5=Nobumichi|last6=Marcus|first6=M. A.|date=2005|title=Zinc mobility and speciation in soil covered by contaminated dredged sediment using micrometer-scale and bulk-averaging X-ray fluorescence, absorption and diffraction techniques|url=https://linkinghub.elsevier.com/retrieve/pii/S0016703704006519|journal=Geochimica et Cosmochimica Acta|language=en|volume=69|issue=5|pages=1173–1198|doi=10.1016/j.gca.2004.08.024|bibcode=2005GeCoA..69.1173I}}</ref><ref name=":1">{{Cite journal|last1=[[Alain Manceau|Manceau]]|first1=A|display-authors=1|last2=Lanson|first2=Martine|last3=Geoffroy|first3=Nicolas|date=2007|title=Natural speciation of Ni, Zn, Ba, and As in ferromanganese coatings on quartz using X-ray fluorescence, absorption, and diffraction|url=https://linkinghub.elsevier.com/retrieve/pii/S0016703706020205|journal=Geochimica et Cosmochimica Acta|language=en|volume=71|issue=1|pages=95–128|doi=10.1016/j.gca.2006.08.036|bibcode=2007GeCoA..71...95M}}</ref><ref>{{Cite journal|last1=[[Alain Manceau|Manceau]]|first1=A.|display-authors=1|last2=Lanson|first2=M.|last3=Takahashi|first3=Y.|date=2014|title=Mineralogy and crystal chemistry of Mn, Fe, Co, Ni, and Cu in a deep-sea Pacific polymetallic nodule|url=https://www.degruyter.com/document/doi/10.2138/am-2014-4742/html|journal=American Mineralogist|language=en|volume=99|issue=10|pages=2068–2083|doi=10.2138/am-2014-4742|bibcode=2014AmMin..99.2068M|s2cid=98025822}}</ref><ref>{{Cite journal|last1=Bodeï|first1=S.|display-authors=1|last2=[[Alain Manceau|Manceau]]|first2=A|last3=Geoffroy|first3=Nicolas|last4=Baronnet|first4=A|last5=Buatier|first5=Martine|date=2007|title=Formation of todorokite from vernadite in Ni-rich hemipelagic sediments|url=https://linkinghub.elsevier.com/retrieve/pii/S0016703707004358|journal=Geochimica et Cosmochimica Acta|language=en|volume=71|issue=23|pages=5698–5716|doi=10.1016/j.gca.2007.07.020|bibcode=2007GeCoA..71.5698B}}</ref><ref>{{Cite journal|last1=Bargar|first1=J.R.|display-authors=1|last2=Fuller|first2=Christopher C.|last3=Marcus|first3=M. A.|last4=Brearley|first4=Adrian J.|last5=Perez De la Rosa|first5=M.|last6=Webb|first6=Samuel M.|last7=Caldwell|first7=Wendel A.|date=2009|title=Structural characterization of terrestrial microbial Mn oxides from Pinal Creek, AZ|url=https://linkinghub.elsevier.com/retrieve/pii/S0016703708006777|journal=Geochimica et Cosmochimica Acta|language=en|volume=73|issue=4|pages=889–910|doi=10.1016/j.gca.2008.10.036|bibcode=2009GeCoA..73..889B|s2cid=59065579 }}</ref> It was discovered at [[Birness]], [[Aberdeenshire]], [[Scotland]]. |
||
== Formation == |
== Formation == |
||
Its precipitation from the oxidation of [[Manganese|Mn(II)]] in oxygenated aqueous solutions is kinetically hindered and slow on mineral surfaces. Biological |
Its precipitation from the oxidation of [[Manganese|Mn(II)]] in oxygenated aqueous solutions is kinetically hindered and slow on mineral surfaces. Biological Mn(II) oxidation is generally fast relative to abiotic Mn(II) oxidation processes, and for this reason the majority of natural birnessites is believed to be produced by [[microorganism]]s, especially [[bacteria]], but also [[Fungus|fungi]].<ref name="Tebo_2004">{{Cite journal|last1=Tebo|first1=B. M.|display-authors=1|last2=Bargar|first2=J.R.|last3=Clement|first3=Brian G.|last4=Dick|first4=Gregory J.|last5=Murray|first5=Karen J.|last6=Parker|first6=Dorothy|last7=Verity|first7=Rebecca|last8=Webb|first8=Samuel M.|date=2004-05-19|title=Biogenic manganese oxides: Properties and mechanisms of formation|url=http://www.annualreviews.org/doi/10.1146/annurev.earth.32.101802.120213|journal=Annual Review of Earth and Planetary Sciences|language=en|volume=32|pages=287–328|doi=10.1146/annurev.earth.32.101802.120213|bibcode=2004AREPS..32..287T}}</ref> |
||
== Composition and structure == |
== Composition and structure == |
||
Birnessite is a [[non-stoichiometric compound]], in which variable amounts of |
Birnessite is a [[non-stoichiometric compound]], in which variable amounts of Mn<sup>4+</sup> ions in the nominal MnO<sub>2</sub>·''n''H<sub>2</sub>O formula either are missing, or are replaced primarily by Mn<sup>3+</sup> ions and secondarily by Mn<sup>2+</sup> ions. Because a solid is overall electrically neutral, birnessite contains foreign cations to balance the net negative charge created by Mn<sup>4+</sup> vacancies and heterovalent Mn substitutions. |
||
Two [[Crystallography|crystallographic]] structures are known, [[Triclinic crystal system|triclinic]] birnessite (TcBi)<ref name=":2">{{Cite journal|last1=Lanson|first1=B|display-authors=1|last2=Drits|first2=V. A.|last3=Feng|first3=Qi|last4=Manceau|first4=A|date=2002|title=Structure of synthetic Na-birnessite: Evidence for a triclinic one-layer unit cell|url=http://dx.doi.org/10.2138/am-2002-11-1215|journal=American Mineralogist|volume=87|issue=11–12|pages=1662–1671|doi=10.2138/am-2002-11-1215|bibcode=2002AmMin..87.1662L|s2cid=53443294}}</ref> and [[Hexagonal crystal family|hexagonal]] birnessite (HBi).<ref name=":3">{{Cite journal|last1=Drits|first1=V. A.|display-authors=1|last2=Silvester|first2=E.|last3=Gorshkov|first3=Anatoli I.|last4=Manceau|first4=A|date=1997|title=Structure of synthetic monoclinic Na-rich birnessite and hexagonal birnessite; I, Results from X-ray diffraction and selected-area electron diffraction|url=http://dx.doi.org/10.2138/am-1997-9-1012|journal=American Mineralogist|volume=82|issue=9–10|pages=946–961|doi=10.2138/am-1997-9-1012|bibcode=1997AmMin..82..946D|s2cid=56030552}}</ref><ref name=":4">{{Cite journal|last1=Silvester|first1=E.|display-authors=1|last2=Manceau|first2=A|last3=Drits|first3=V. A.|date=1997|title=Structure of synthetic monoclinic Na-rich birnessite and hexagonal birnessite; II, Results from chemical studies and EXAFS spectroscopy|url=http://dx.doi.org/10.2138/am-1997-9-1013|journal=American Mineralogist|volume=82|issue=9–10|pages=962–978|doi=10.2138/am-1997-9-1013|bibcode=1997AmMin..82..962S|s2cid=55969753}}</ref><ref name=":5">{{Cite journal|last1=Lanson|first1=B|display-authors=1|last2=Drits|first2=V. A.|last3=Silvester|first3=E.|last4=Manceau|first4=A|date=2000|title=Structure of H-exchanged hexagonal birnessite and its mechanism of formation from Na-rich monoclinic buserite at low pH|url=http://dx.doi.org/10.2138/am-2000-5-625|journal=American Mineralogist|volume=85|issue=5–6|pages=826–838|doi=10.2138/am-2000-5-625|bibcode=2000AmMin..85..826L|s2cid=55586944}}</ref> The two of them consist of layers of edge-sharing MnO<sub>6</sub> octahedra separated by one or two layers of |
Two [[Crystallography|crystallographic]] structures are known, [[Triclinic crystal system|triclinic]] birnessite (TcBi)<ref name=":2">{{Cite journal|last1=Lanson|first1=B|display-authors=1|last2=Drits|first2=V. A.|last3=Feng|first3=Qi|last4=[[Alain Manceau|Manceau]]|first4=A|date=2002|title=Structure of synthetic Na-birnessite: Evidence for a triclinic one-layer unit cell|url=http://dx.doi.org/10.2138/am-2002-11-1215|journal=American Mineralogist|volume=87|issue=11–12|pages=1662–1671|doi=10.2138/am-2002-11-1215|bibcode=2002AmMin..87.1662L|s2cid=53443294}}</ref> and [[Hexagonal crystal family|hexagonal]] birnessite (HBi).<ref name=":3">{{Cite journal|last1=Drits|first1=V. A.|display-authors=1|last2=Silvester|first2=E.|last3=Gorshkov|first3=Anatoli I.|last4=[[Alain Manceau|Manceau]]|first4=A|date=1997|title=Structure of synthetic monoclinic Na-rich birnessite and hexagonal birnessite; I, Results from X-ray diffraction and selected-area electron diffraction|url=http://dx.doi.org/10.2138/am-1997-9-1012|journal=American Mineralogist|volume=82|issue=9–10|pages=946–961|doi=10.2138/am-1997-9-1012|bibcode=1997AmMin..82..946D|s2cid=56030552}}</ref><ref name=":4">{{Cite journal|last1=Silvester|first1=E.|display-authors=1|last2=[[Alain Manceau|Manceau]]|first2=A|last3=Drits|first3=V. A.|date=1997|title=Structure of synthetic monoclinic Na-rich birnessite and hexagonal birnessite; II, Results from chemical studies and EXAFS spectroscopy|url=http://dx.doi.org/10.2138/am-1997-9-1013|journal=American Mineralogist|volume=82|issue=9–10|pages=962–978|doi=10.2138/am-1997-9-1013|bibcode=1997AmMin..82..962S|s2cid=55969753}}</ref><ref name=":5">{{Cite journal|last1=Lanson|first1=B|display-authors=1|last2=Drits|first2=V. A.|last3=Silvester|first3=E.|last4=[[Alain Manceau|Manceau]]|first4=A|date=2000|title=Structure of H-exchanged hexagonal birnessite and its mechanism of formation from Na-rich monoclinic buserite at low pH|url=http://dx.doi.org/10.2138/am-2000-5-625|journal=American Mineralogist|volume=85|issue=5–6|pages=826–838|doi=10.2138/am-2000-5-625|bibcode=2000AmMin..85..826L|s2cid=55586944}}</ref> The two of them consist of layers of edge-sharing MnO<sub>6</sub> octahedra separated by one or two layers of water molecules. The one-water layer compounds have a characteristic ~7 [[Angstrom|Å]] repeat in the layer stacking direction, and addition of a second water layer expands the layer spacing to ~10 Å. The 10 Å form is named [[buserite]]. |
||
The layer composition of TcBi is typically Mn<sup>4+</sup><sub>0.69</sub>Mn<sup>3+</sup><sub>0.31</sub>. The Mn<sup>3+</sup>O<sub>6</sub> and Mn<sup>4+</sup>O<sub>6</sub> octahedra are fully ordered in raws in the MnO<sub>2</sub> layers, such that every |
The layer composition of TcBi is typically Mn<sup>4+</sup><sub>0.69</sub>Mn<sup>3+</sup><sub>0.31</sub>. The Mn<sup>3+</sup>O<sub>6</sub> and Mn<sup>4+</sup>O<sub>6</sub> octahedra are fully ordered in raws in the MnO<sub>2</sub> layers, such that every Mn<sup>3+</sup>-rich row alternates with two Mn<sup>4+</sup>-rich rows.<sup>10,11</sup> The layer charge is offset by alkaline and alkali-earth cations (e.g., Na, K, Ca, Ba) into the interlayer region along with water molecules, and therefore TcBi has a [[cation-exchange capacity]]. A typical chemical formula of [[Sodium|Na]]-exchanged TcBi is Na<sub>0.31</sub>(Mn<sup>4+</sup><sub>0.69</sub> Mn<sup>3+</sup><sub>0.31</sub>)O<sub>2</sub>·0.4H<sub>2</sub>O.<ref name=":2" /> |
||
The layer structure of HBi differs from that of TcBi by the presence of octahedral |
The layer structure of HBi differs from that of TcBi by the presence of octahedral Mn<sup>4+</sup> vacancies (Vac). The chemical formula of synthetic HBi depends on [[pH]]. The generic formula is H<sup>+</sup>''<sub>x</sub>'' Mn<sup>3+</sup>''<sub>y</sub>'' Mn<sup>2+</sup>''<sub>z</sub>'' (Mn<sup>4+</sup>''<sub>u</sub>'' Mn<sup>3+</sup>''<sub>v</sub>''Vac''<sub>w</sub>'')O<sub>2</sub>, with ''x'' + 3''y'' + 2''z'' = ''v'' + 4''w'' for neutrality.<ref name=":3" /><ref name=":4" /><ref name=":5" /> |
||
The MnO<sub>2</sub> layers are stacked periodically in synthetic |
The MnO<sub>2</sub> layers are stacked periodically in synthetic triclinic and hexagonal birnessite [[crystal]]s. It is, however, rarely the case in natural materials. In addition to being chemically complex, natural birnessite crystals are structurally disordered with respect to the layer stacking and the flatness of the layers.<ref>{{Cite journal|last1=[[Alain Manceau|Manceau]]|first1=A|display-authors=1|last2=Marcus|first2=Matthew A.|last3=Grangeon|first3=S.|last4=Lanson|first4=M.|last5=Lanson|first5=B.|last6=Gaillot|first6=A.-C.|last7=Skanthakumar|first7=S.|last8=Soderholm|first8=L.|date=2013-02-01|title=Short-range and long-range order of phyllomanganate nanoparticles determined using high-energy X-ray scattering|url=http://scripts.iucr.org/cgi-bin/paper?S0021889812047917|journal=Journal of Applied Crystallography|volume=46|issue=|pages=193–209|doi=10.1107/S0021889812047917|s2cid=56356250|issn=}}</ref> A natural birnessite crystal may contain only a few layers, and they are often bent and always imperfectly stacked with orientational and translational loss of registry. The stacking disorder is referred to as "turbostratic" when the layers are oriented completely at random. Natural birnessite with turbostratically stacked layers is named vernadite,<ref>{{Cite journal|last=Giovanoli|first=R.|date=1980|title=Vernadite is random-stacked birnessite|url=http://dx.doi.org/10.1007/bf00206520|journal=Mineralium Deposita|volume=15|issue=2|pages=251–253|doi=10.1007/bf00206520|bibcode=1980MinDe..15..251G|s2cid=104363781}}</ref><ref>{{Cite journal|last1=[[Alain Manceau|Manceau]]|first1=A.|display-authors=1|last2=Combes|first2=J. M.|date=1988|title=Structure of Mn and Fe oxides and oxyhydroxides: A topological approach by EXAFS|url=http://dx.doi.org/10.1007/bf00307518|journal=Physics and Chemistry of Minerals|volume=15|issue=3|pages=283–295|doi=10.1007/bf00307518|bibcode=1988PCM....15..283M|s2cid=97465847}}</ref><ref>{{Cite journal|last=Hochella|first=M. F.|date=2005|title=Environmentally important, poorly crystalline Fe/Mn hydrous oxides: Ferrihydrite and a possibly new vernadite-like mineral from the Clark Fork River Superfund Complex|url=http://dx.doi.org/10.2138/am.2005.1591|journal=American Mineralogist|volume=90|issue=4|pages=718–724|doi=10.2138/am.2005.1591|bibcode=2005AmMin..90..718H|s2cid=54664549}}</ref> and the synthetic analog is named δ-MnO<sub>2</sub>. The layer spacing of vernadite can be also ~7 Å or ~10 Å, and interstratification of the two types of layers has been observed on quartz coatings<ref name=":1" /> and in ferromanganese crusts.<ref>{{Cite journal|last1=Lee|first1=S|display-authors=1|last2=Xu|first2=Hfang|last3=Xu|first3=Wenqian|last4=Sun|first4=Xiaoming|date=2019|title=The structure and crystal chemistry of vernadite in ferromanganese crusts|url=https://scripts.iucr.org/cgi-bin/paper?S2052520619006528|journal=Acta Crystallographica Section B|volume=75|issue=4|pages=591–598|doi=10.1107/S2052520619006528|pmid=32830716|osti=1559941|s2cid=202084338}}</ref> |
||
== Surface reactivity == |
== Surface reactivity == |
||
The +4 charge deficit of a vacancy can be balanced by a large variety of interlayer cations forming [[Inner sphere complex|inner-sphere complexes]] above and below the vacancies (e.g., [[Calcium|Ca]], [[Copper|Cu]], [[Zinc|Zn]], [[Lead|Pb]], [[Cadmium|Cd]], [[Thallium|Tl]]).<ref name=":0" /><ref>{{Cite journal|last1=Lanson|first1=B|display-authors=1|last2=Drits|first2=V. A.|last3=Gaillot|first3=Anne-Claire|last4=Silvester|first4=E.|last5=Plançon|first5=A|last6=Manceau|first6=A|date=2002|title=Structure of heavy-metal sorbed birnessite: Part 1. Results from X-ray diffraction|url=http://dx.doi.org/10.2138/am-2002-11-1213|journal=American Mineralogist|volume=87|issue=11–12|pages=1631–1645|doi=10.2138/am-2002-11-1213|bibcode=2002AmMin..87.1631L|s2cid=52255943}}</ref><ref>{{Cite journal|last1=Drits|first1=V. A.|display-authors=1|last2=Lanson|first2=B|last3=Bougerol-Chaillout|first3=Catherine|last4=Gorshkov|first4=Anatoli I.|last5=Manceau|first5=A|date=2002|title=Structure of heavy-metal sorbed birnessite: Part 2. Results from electron diffraction|url=http://dx.doi.org/10.2138/am-2002-11-1214|journal=American Mineralogist|volume=87|issue=11–12|pages=1646–1661|doi=10.2138/am-2002-11-1214|bibcode=2002AmMin..87.1646D|s2cid=52241298}}</ref><ref>{{Cite journal|last1=Manceau|first1=A|display-authors=1|last2=Lanson|first2=B|last3=Drits|first3=V. A.|date=2002|title=Structure of heavy metal sorbed birnessite. Part III: Results from powder and polarized extended X-ray absorption fine structure spectroscopy|url=http://dx.doi.org/10.1016/s0016-7037(02)00869-4|journal=Geochimica et Cosmochimica Acta|volume=66|issue=15|pages=2639–2663|doi=10.1016/s0016-7037(02)00869-4|bibcode=2002GeCoA..66.2639M}}</ref><ref>{{Cite journal|last1=Marcus|first1=M. A|display-authors=1|last2=Manceau|first2=A|last3=Kersten|first3=Michael|date=2004|title=Mn, Fe, Zn and As speciation in a fast-growing ferromanganese marine nodule|url=http://dx.doi.org/10.1016/j.gca.2004.01.015|journal=Geochimica et Cosmochimica Acta|volume=68|issue=14|pages=3125–3136|doi=10.1016/j.gca.2004.01.015|bibcode=2004GeCoA..68.3125M}}</ref><ref>{{Cite journal|last1=Takahashi|first1=Y|display-authors=1|last2=Manceau|first2=A|last3=Geoffroy|first3=Nicolas|last4=Marcus|first4=M. A.|last5=Usui|first5=Akira|date=2007|title=Chemical and structural control of the partitioning of Co, Ce, and Pb in marine ferromanganese oxides|url=http://dx.doi.org/10.1016/j.gca.2006.11.016|journal=Geochimica et Cosmochimica Acta|volume=71|issue=4|pages=984–1008|doi=10.1016/j.gca.2006.11.016|bibcode=2007GeCoA..71..984T}}</ref><ref>{{Cite journal|last1=Lanson|first1=B|display-authors=1|last2=Marcus|first2=M. A.|last3=Fakra|first3=Sirine|last4=Panfili|first4=Frédéric|last5=Geoffroy|first5=Nicolas|last6=Manceau|first6=A|date=2008|title=Formation of Zn–Ca phyllomanganate nanoparticles in grass roots|url=http://dx.doi.org/10.1016/j.gca.2008.02.022|journal=Geochimica et Cosmochimica Acta|volume=72|issue=10|pages=2478–2490|doi=10.1016/j.gca.2008.02.022|bibcode=2008GeCoA..72.2478L}}</ref><ref name="grass">{{Cite journal|last1=Wick|first1=S|display-authors=1|last2=Peña|first2=Jasquelin|last3=Voegelin|first3=Andreas|date=2019|title=Thallium Sorption onto Manganese Oxides |
The +4 charge deficit of a vacancy can be balanced by a large variety of interlayer cations forming [[Inner sphere complex|inner-sphere complexes]] above and below the vacancies (e.g., [[Calcium|Ca]], [[Copper|Cu]], [[Zinc|Zn]], [[Lead|Pb]], [[Cadmium|Cd]], [[Thallium|Tl]]).<ref name=":0" /><ref>{{Cite journal|last1=Lanson|first1=B|display-authors=1|last2=Drits|first2=V. A.|last3=Gaillot|first3=Anne-Claire|last4=Silvester|first4=E.|last5=Plançon|first5=A|last6=[[Alain Manceau|Manceau]]|first6=A|date=2002|title=Structure of heavy-metal sorbed birnessite: Part 1. Results from X-ray diffraction|url=http://dx.doi.org/10.2138/am-2002-11-1213|journal=American Mineralogist|volume=87|issue=11–12|pages=1631–1645|doi=10.2138/am-2002-11-1213|bibcode=2002AmMin..87.1631L|s2cid=52255943}}</ref><ref>{{Cite journal|last1=Drits|first1=V. A.|display-authors=1|last2=Lanson|first2=B|last3=Bougerol-Chaillout|first3=Catherine|last4=Gorshkov|first4=Anatoli I.|last5=[[Alain Manceau|Manceau]]|first5=A|date=2002|title=Structure of heavy-metal sorbed birnessite: Part 2. Results from electron diffraction|url=http://dx.doi.org/10.2138/am-2002-11-1214|journal=American Mineralogist|volume=87|issue=11–12|pages=1646–1661|doi=10.2138/am-2002-11-1214|bibcode=2002AmMin..87.1646D|s2cid=52241298}}</ref><ref>{{Cite journal|last1=[[Alain Manceau|Manceau]]|first1=A|display-authors=1|last2=Lanson|first2=B|last3=Drits|first3=V. A.|date=2002|title=Structure of heavy metal sorbed birnessite. Part III: Results from powder and polarized extended X-ray absorption fine structure spectroscopy|url=http://dx.doi.org/10.1016/s0016-7037(02)00869-4|journal=Geochimica et Cosmochimica Acta|volume=66|issue=15|pages=2639–2663|doi=10.1016/s0016-7037(02)00869-4|bibcode=2002GeCoA..66.2639M}}</ref><ref>{{Cite journal|last1=Marcus|first1=M. A|display-authors=1|last2=[[Alain Manceau|Manceau]]|first2=A|last3=Kersten|first3=Michael|date=2004|title=Mn, Fe, Zn and As speciation in a fast-growing ferromanganese marine nodule|url=http://dx.doi.org/10.1016/j.gca.2004.01.015|journal=Geochimica et Cosmochimica Acta|volume=68|issue=14|pages=3125–3136|doi=10.1016/j.gca.2004.01.015|bibcode=2004GeCoA..68.3125M|s2cid=33694906 }}</ref><ref>{{Cite journal|last1=Takahashi|first1=Y|display-authors=1|last2=[[Alain Manceau|Manceau]]|first2=A|last3=Geoffroy|first3=Nicolas|last4=Marcus|first4=M. A.|last5=Usui|first5=Akira|date=2007|title=Chemical and structural control of the partitioning of Co, Ce, and Pb in marine ferromanganese oxides|url=http://dx.doi.org/10.1016/j.gca.2006.11.016|journal=Geochimica et Cosmochimica Acta|volume=71|issue=4|pages=984–1008|doi=10.1016/j.gca.2006.11.016|bibcode=2007GeCoA..71..984T}}</ref><ref>{{Cite journal|last1=Lanson|first1=B|display-authors=1|last2=Marcus|first2=M. A.|last3=Fakra|first3=Sirine|last4=Panfili|first4=Frédéric|last5=Geoffroy|first5=Nicolas|last6=[[Alain Manceau|Manceau]]|first6=A|date=2008|title=Formation of Zn–Ca phyllomanganate nanoparticles in grass roots|url=http://dx.doi.org/10.1016/j.gca.2008.02.022|journal=Geochimica et Cosmochimica Acta|volume=72|issue=10|pages=2478–2490|doi=10.1016/j.gca.2008.02.022|bibcode=2008GeCoA..72.2478L|s2cid=16075545}}</ref><ref name="grass">{{Cite journal|last1=Wick|first1=S|display-authors=1|last2=Peña|first2=Jasquelin|last3=Voegelin|first3=Andreas|date=2019|title=Thallium Sorption onto Manganese Oxides|journal=Environmental Science & Technology|language=en|volume=53|issue=22|pages=13168–13178|doi=10.1021/acs.est.9b04454|pmid=31674774|bibcode=2019EnST...5313168W|s2cid=207815275|doi-access=free|hdl=20.500.11850/383584|hdl-access=free}}</ref><ref>{{Cite journal|last1=[[Alain Manceau|Manceau]]|first1=A.|display-authors=1|last2=Simionovici|first2=A.|date=2022|title=Crystal Chemistry of Thallium in Marine Ferromanganese Deposits|url=https://doi.org/10.1021/acsearthspacechem.1c00447|journal=ACS Earth and Space Chemistry|volume=6|issue=5 |pages=1269–1285|doi=10.1021/acsearthspacechem.1c00447|bibcode=2022ESC.....6.1269M }}</ref> The relative stability of the interlayer cations has been evaluated experimentally<ref>{{Cite journal|last1=Wang|first1=Y|display-authors=1|last2=Feng|first2=Xionghan|last3=Villalobos|first3=Mario|last4=Tan|first4=Wenfeng|last5=Liu|first5=Fan|date=2012|title=Sorption behavior of heavy metals on birnessite: Relationship with its Mn average oxidation state and implications for types of sorption sites|url=http://dx.doi.org/10.1016/j.chemgeo.2011.11.001|journal=Chemical Geology|volume=292-293|pages=25–34|doi=10.1016/j.chemgeo.2011.11.001|bibcode=2012ChGeo.292...25W}}</ref><ref>{{Cite journal|last=Murray|first=J. W.|date=1975|title=The interaction of metal ions at the manganese dioxide-solution interface|url=https://linkinghub.elsevier.com/retrieve/pii/0016703775901039|journal=Geochimica et Cosmochimica Acta|language=en|volume=39|issue=4|pages=505–519|doi=10.1016/0016-7037(75)90103-9|bibcode=1975GeCoA..39..505M}}</ref> and theoretically by surface complexation modeling<ref name="Appelo_Postma_1999">{{Cite journal|last1=Appelo|first1=C.A.J.|display-authors=1|last2=Postma|first2=D.|date=1999|title=A consistent model for surface complexation on birnessite (δ-MnO<sub>2</sub>) and its application to a column experiment|url=http://dx.doi.org/10.1016/s0016-7037(99)00231-8|journal=Geochimica et Cosmochimica Acta|volume=63|issue=19–20|pages=3039–3048|doi=10.1016/s0016-7037(99)00231-8|bibcode=1999GeCoA..63.3039A}}</ref> and computational chemistry.<ref>{{Cite journal|last1=Kwon|first1=K. D.|display-authors=1|last2=Refson|first2=Keith|last3=Sposito|first3=Garrison|date=2013|title=Understanding the trends in transition metal sorption by vacancy sites in birnessite|url=http://dx.doi.org/10.1016/j.gca.2012.08.038|journal=Geochimica et Cosmochimica Acta|volume=101|pages=222–232|doi=10.1016/j.gca.2012.08.038|bibcode=2013GeCoA.101..222K}}</ref><ref>{{Cite journal|last1=[[Alain Manceau|Manceau]]|first1=A|display-authors=1|last2=Steinmann|first2=Stephan N.|date=2021|title=Nature of High- and Low-Affinity Metal Surface Sites on Birnessite Nanosheets|url=https://pubs.acs.org/doi/10.1021/acsearthspacechem.0c00278|journal=ACS Earth and Space Chemistry|language=en|volume=5|issue=1|pages=66–76|doi=10.1021/acsearthspacechem.0c00278|bibcode=2021ESC.....5...66M|s2cid=234304816}}</ref> Pb<sup>2+</sup> has the highest stability at the HBi surface, and the high geochemical affinity of Pb<sup>2+</sup> for birnessite probably explains its billion-fold enrichment in marine ferromanganese deposits compared to [[seawater]], which surpasses those of all other elements.<ref name=":6">{{Citation|last1=Hein|first1=J.R.|title=Deep-Ocean Ferromanganese Crusts and Nodules|date=2014|url=http://dx.doi.org/10.1016/b978-0-08-095975-7.01111-6|work=Treatise on Geochemistry|pages=273–291|publisher=Elsevier|access-date=2021-09-26|display-authors=1|last2=Koschinsky|first2=A.|doi=10.1016/b978-0-08-095975-7.01111-6|isbn=9780080983004}}</ref> |
||
[[Transition metal]] cations sorbed on vacancies were also observed to enter the underlying vacancies and become incorporated into the MnO<sub>2</sub> layer, when their effective ionic radius<ref>{{Cite journal|last=Shannon|first=R. D.|date=1976|title=Revised effective ionic radii and systematic studies of interatomic distances in halides and chalcogenides|url=http://dx.doi.org/10.1107/s0567739476001551|journal=Acta Crystallographica Section A|volume=32|issue=5|pages=751–767|doi=10.1107/s0567739476001551|bibcode=1976AcCrA..32..751S}}</ref> is close to that of |
[[Transition metal]] cations sorbed on vacancies were also observed to enter the underlying vacancies and become incorporated into the MnO<sub>2</sub> layer, when their effective ionic radius<ref>{{Cite journal|last=Shannon|first=R. D.|date=1976|title=Revised effective ionic radii and systematic studies of interatomic distances in halides and chalcogenides|url=http://dx.doi.org/10.1107/s0567739476001551|journal=Acta Crystallographica Section A|volume=32|issue=5|pages=751–767|doi=10.1107/s0567739476001551|bibcode=1976AcCrA..32..751S}}</ref> is close to that of Mn<sup>4+</sup> (r = 0.53 Å). For example, Zn<sup>2+</sup> (r = 0.74 Å) was never observed to enter a vacancy site, while Ni<sup>2+</sup> (r = 0.69 Å) partly enters, and Co<sup>3+</sup> (r = 0.54 Å) always does.<ref name=":1" /><ref name="Manceau_1997">{{Cite journal|last1=[[Alain Manceau|Manceau]]|first1=A|display-authors=1|last2=Drits|first2=V. A.|last3=Silvester|first3=E.|last4=Bartoli|first4=Celine|last5=Lanson|first5=B|date=1997|title=Structural mechanism of Co<sup>2+</sup> oxidation by the phyllomanganate buserite|url=http://dx.doi.org/10.2138/am-1997-11-1213|journal=American Mineralogist|volume=82|issue=11–12|pages=1150–1175|doi=10.2138/am-1997-11-1213|bibcode=1997AmMin..82.1150M|s2cid=54923713}}</ref><ref>{{Cite journal|last1=Peacock|first1=C. L.|display-authors=1|last2=Sherman|first2=D. M.|date=2007|title=Crystal-chemistry of Ni in marine ferromanganese crusts and nodules|url=http://dx.doi.org/10.2138/am.2007.2378|journal=American Mineralogist|volume=92|issue=7|pages=1087–1092|doi=10.2138/am.2007.2378|bibcode=2007AmMin..92.1087P|s2cid=98074547}}</ref> Octahedral high-spin [[Cobalt|Co<sup>2+</sup>]] (r = 0.74 Å) sorbed on a vacancy is transformed into a smaller tetrahedral complex (r = 0.58 Å) to penetrate into the octahedral Mn vacancy, and is subsequently converted to the low-spin state before being oxidized to Co<sup>3+</sup> by Mn<sup>4+</sup>, which is reduced to Mn<sup>3+</sup>.<ref>{{Cite journal|last1=[[Alain Manceau|Manceau]]|first1=A.|last2=Steinmann|first2=S.|date=2022|title=Density Functional Theory Modeling of the Oxidation Mechanism of Co(II) by Birnessite|url=https://doi.org/10.1021/acsearthspacechem.2c00122|journal=ACS Earth and Space Chemistry|volume=6|issue=8 |pages=2063–2075|doi=10.1021/acsearthspacechem.2c00122|bibcode=2022ESC.....6.2063M |s2cid=251086409 }}</ref> The surface-catalyzed, redox-driven uptake of Co leads also to a billion-fold enrichment in marine ferromanganese deposits compared to Co between ferromanganese deposits and Pb<sup>2+</sup>.<ref name=":6" /> |
||
== Properties and applications == |
== Properties and applications == |
||
In nature, photosynthetic organisms use the high oxidative ability of birnessite-type Mn<sub>4</sub>CaO<sub>5</sub> clusters to oxidize |
In nature, photosynthetic organisms use the high oxidative ability of birnessite-type Mn<sub>4</sub>CaO<sub>5</sub> clusters to oxidize water into molecular oxygen through the [[photosystem II]] membrane protein complex.<ref>{{Cite journal|last1=Chernev|first1=P.|display-authors=1|last2=Fischer|first2=Sophie|last3=Hoffmann|first3=Jutta|last4=Oliver|first4=Nicholas|last5=Assunção|first5=Ricardo|last6=Yu|first6=Boram|last7=Burnap|first7=Robert L.|last8=Zaharieva|first8=Ivelina|last9=Nürnberg|first9=Dennis J.|last10=Haumann|first10=Michael|last11=Dau|first11=Holger|date=2021|title=Publisher Correction: Light-driven formation of manganese oxide by today's photosystem II supports evolutionarily ancient manganese-oxidizing photosynthesis|url=http://dx.doi.org/10.1038/s41467-020-20868-9|journal=Nature Communications|volume=12|issue=1|page=419|doi=10.1038/s41467-020-20868-9|pmid=33436628|pmc=7804171}}</ref> |
||
Because of their [[Semiconductor|semiconducting]] properties, birnessite-type materials are used in a variety of areas, including [[catalysis]] and [[Electrochemistry|electrochemical]] energy storage ([[Electric battery|batteries]] and [[pseudocapacitor]]s). The ordering of the |
Because of their [[Semiconductor|semiconducting]] properties, birnessite-type materials are used in a variety of areas, including [[catalysis]] and [[Electrochemistry|electrochemical]] energy storage ([[Electric battery|batteries]] and [[pseudocapacitor]]s). The ordering of the Mn<sup>3+</sup> cations in triclinic birnessite, and the Mn<sup>4+</sup> vacancies in hexagonal birnessite, both reduce the [[band gap]],<ref>{{Cite journal|last1=Lucht|first1=K. P.|display-authors=1|last2=Mendoza-Cortes|first2=Jose L.|date=2015|title=Birnessite: A Layered Manganese Oxide To Capture Sunlight for Water-Splitting Catalysis|url=http://dx.doi.org/10.1021/acs.jpcc.5b07860|journal=The Journal of Physical Chemistry C|volume=119|issue=40|pages=22838–22846|doi=10.1021/acs.jpcc.5b07860}}</ref><ref>{{Cite journal|last1=Kwon|first1=K. D.|display-authors=1|last2=Refson|first2=Keith|last3=Sposito|first3=Garrison|date=2008|title=Defect-Induced Photoconductivity in Layered Manganese Oxides: A Density Functional Theory Study|url=http://dx.doi.org/10.1103/physrevlett.100.146601|journal=Physical Review Letters|volume=100|issue=14|page=146601|doi=10.1103/physrevlett.100.146601|pmid=18518059|bibcode=2008PhRvL.100n6601K|s2cid=2146794 }}</ref><ref>{{Cite journal|last1=Gao|first1=P.|display-authors=1|last2=Chen|first2=Zhen|last3=Gong|first3=Yuxuan|last4=Zhang|first4=Rui|last5=Liu|first5=H|last6=Tang|first6=Pei|last7=Chen|first7=Xiaohua|last8=Passerini|first8=Stefano|last9=Liu|first9=Jilei|date=2020|title=The Role of Cation Vacancies in Electrode Materials for Enhanced Electrochemical Energy Storage: Synthesis, Advanced Characterization, and Fundamentals|url=http://dx.doi.org/10.1002/aenm.201903780|journal=Advanced Energy Materials|volume=10|issue=14|pages=1903780|doi=10.1002/aenm.201903780|s2cid=214006754}}</ref> and therefore enhance [[Electrical resistivity and conductivity|electrical conductivity]]. Half-[[Metallic bonding|metallic]] behavior was observed for MnO<sub>2</sub> nanosheets with Mn vacancies, rendering them promising candidates for applications in [[spintronics]].<ref>{{Cite journal|last1=Wang|first1=H|display-authors=1|last2=Zhang|first2=Jiajia|last3=Hang|first3=Xudong|last4=Zhang|first4=Xiaodong|last5=Xie|first5=Junfeng|last6=Pan|first6=Bicai|last7=Xie|first7=Yi|date=2014|title=Half-Metallicity in Single-Layered Manganese Dioxide Nanosheets by Defect Engineering|url=http://dx.doi.org/10.1002/ange.201410031|journal=Angewandte Chemie|volume=127|issue=4|pages=1211–1215|doi=10.1002/ange.201410031}}</ref> |
||
Birnessite is able to break down [[prions]] via oxidation.<ref>{{Cite journal|last1=Russo|first1=F.|display-authors=1|last2=Johnson|first2=C. J.|last3=Johnson|first3=C. J.|last4=McKenzie|first4=D.|last5=Aiken|first5=J. M.|last6=Pedersen|first6=J. A.|date=2009|title=Pathogenic prion protein is degraded by a manganese oxide mineral found in soils|journal=Journal of General Virology|volume=90|issue=Pt 1|pages=275–280|doi=10.1099/vir.0.003251-0|pmid=19088299|doi-access=free}}</ref> How well this process works outside the laboratory is unclear. |
Birnessite is able to break down [[prions]] via oxidation.<ref>{{Cite journal|last1=Russo|first1=F.|display-authors=1|last2=Johnson|first2=C. J.|last3=Johnson|first3=C. J.|last4=McKenzie|first4=D.|last5=Aiken|first5=J. M.|last6=Pedersen|first6=J. A.|date=2009|title=Pathogenic prion protein is degraded by a manganese oxide mineral found in soils|journal=Journal of General Virology|volume=90|issue=Pt 1|pages=275–280|doi=10.1099/vir.0.003251-0|pmid=19088299|doi-access=free}}</ref> How well this process works outside the laboratory is unclear. |
||
| Line 69: | Line 69: | ||
<gallery widths="250" heights="160" perrow="5"> |
<gallery widths="250" heights="160" perrow="5"> |
||
File:Baltic nodule.jpg|X-ray fluorescence tricolor map of a ferromanganese nodule from the Baltic |
File:Baltic nodule.jpg|[[X-ray fluorescence]] tricolor map of a [[ferromanganese nodules|ferromanganese nodule]] from the [[Baltic Sea]]. |
||
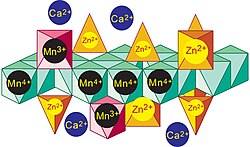
File:Me-vernadite root coating.jpg|Idealized structure for Zn-rich vernadite nanosheets precipitated in the epidermis of grass roots. |
File:Me-vernadite root coating.jpg|Idealized structure for Zn-rich vernadite nanosheets precipitated in the epidermis of grass roots. |
||
File:D-MnO2 TEM image.jpg|High-magnification electron image of |
File:D-MnO2 TEM image.jpg|High-magnification [[transmission electron microscope]] (TEM) image of δ-{{chem2|MnO2}} nano-crystals viewed parallel and perpendicular to the layer plane. |
||
File:DBi10 cylindrical bent.jpg|Structure of a cylindrically bent layer of |
File:DBi10 cylindrical bent.jpg|Structure of a cylindrically bent layer of δ-{{chem2|MnO2}} nanosheet. |
||
File:Structure of a spherically bent layer of d-MnO2.jpg|Structure of a spherically bent layer of |
File:Structure of a spherically bent layer of d-MnO2.jpg|Structure of a spherically bent layer of δ-{{chem2|MnO2}} nanosheet. |
||
</gallery> |
</gallery> |
||
| Line 79: | Line 79: | ||
Other manganese oxides: |
Other manganese oxides: |
||
* [[Buserite]] |
* [[Buserite]] |
||
| ⚫ | |||
* [[Psilomelane]] |
* [[Psilomelane]] |
||
| ⚫ | |||
* [[Ramsdellite]] |
|||
* [[Todorokite]] |
|||
== References == |
== References == |
||
Latest revision as of 13:04, 22 May 2024
| Birnessite | |
|---|---|
 | |
| General | |
| Category | Oxide mineral |
| Formula (repeating unit) | MnO2·nH2O δ-MnO2 |
| IMA symbol | Bir[1] |
| Crystal system | Triclinic or Hexagonal |
| Identification | |
| Color | Dark brown to black |
| Crystal habit | Extremely finely crystalline |
| Cleavage | [001] perfect |
| Mohs scale hardness | 1.5 |
| Luster | Sub-metallic, dull |
| Diaphaneity | Nearly opaque |
| Specific gravity | 3.0 |
| Optical properties | Uniaxial (−) |
| Refractive index | nω = 1.730 nε = 1.690 |
| Birefringence | δ = 0.040 |
| Other characteristics | Identification by optical properties is impossible. |
| References | [2][3][4] |
Birnessite (nominally MnO2·nH2O), also known as δ-MnO2, is a hydrous manganese dioxide mineral with a chemical formula of Na0.7Ca0.3Mn7O14·2.8H2O.[5] It is the main manganese mineral species at the Earth's surface, and commonly occurs as fine-grained, poorly crystallized aggregates in soils, sediments, grain and rock coatings (e.g., desert varnish), and marine ferromanganese nodules and crusts.[6][7][8][9][10][11][12] It was discovered at Birness, Aberdeenshire, Scotland.
Formation
[edit]Its precipitation from the oxidation of Mn(II) in oxygenated aqueous solutions is kinetically hindered and slow on mineral surfaces. Biological Mn(II) oxidation is generally fast relative to abiotic Mn(II) oxidation processes, and for this reason the majority of natural birnessites is believed to be produced by microorganisms, especially bacteria, but also fungi.[13]
Composition and structure
[edit]Birnessite is a non-stoichiometric compound, in which variable amounts of Mn4+ ions in the nominal MnO2·nH2O formula either are missing, or are replaced primarily by Mn3+ ions and secondarily by Mn2+ ions. Because a solid is overall electrically neutral, birnessite contains foreign cations to balance the net negative charge created by Mn4+ vacancies and heterovalent Mn substitutions.
Two crystallographic structures are known, triclinic birnessite (TcBi)[14] and hexagonal birnessite (HBi).[15][16][17] The two of them consist of layers of edge-sharing MnO6 octahedra separated by one or two layers of water molecules. The one-water layer compounds have a characteristic ~7 Å repeat in the layer stacking direction, and addition of a second water layer expands the layer spacing to ~10 Å. The 10 Å form is named buserite.
The layer composition of TcBi is typically Mn4+0.69Mn3+0.31. The Mn3+O6 and Mn4+O6 octahedra are fully ordered in raws in the MnO2 layers, such that every Mn3+-rich row alternates with two Mn4+-rich rows.10,11 The layer charge is offset by alkaline and alkali-earth cations (e.g., Na, K, Ca, Ba) into the interlayer region along with water molecules, and therefore TcBi has a cation-exchange capacity. A typical chemical formula of Na-exchanged TcBi is Na0.31(Mn4+0.69 Mn3+0.31)O2·0.4H2O.[14]
The layer structure of HBi differs from that of TcBi by the presence of octahedral Mn4+ vacancies (Vac). The chemical formula of synthetic HBi depends on pH. The generic formula is H+x Mn3+y Mn2+z (Mn4+u Mn3+vVacw)O2, with x + 3y + 2z = v + 4w for neutrality.[15][16][17]
The MnO2 layers are stacked periodically in synthetic triclinic and hexagonal birnessite crystals. It is, however, rarely the case in natural materials. In addition to being chemically complex, natural birnessite crystals are structurally disordered with respect to the layer stacking and the flatness of the layers.[18] A natural birnessite crystal may contain only a few layers, and they are often bent and always imperfectly stacked with orientational and translational loss of registry. The stacking disorder is referred to as "turbostratic" when the layers are oriented completely at random. Natural birnessite with turbostratically stacked layers is named vernadite,[19][20][21] and the synthetic analog is named δ-MnO2. The layer spacing of vernadite can be also ~7 Å or ~10 Å, and interstratification of the two types of layers has been observed on quartz coatings[9] and in ferromanganese crusts.[22]
Surface reactivity
[edit]The +4 charge deficit of a vacancy can be balanced by a large variety of interlayer cations forming inner-sphere complexes above and below the vacancies (e.g., Ca, Cu, Zn, Pb, Cd, Tl).[8][23][24][25][26][27][28][29][30] The relative stability of the interlayer cations has been evaluated experimentally[31][32] and theoretically by surface complexation modeling[33] and computational chemistry.[34][35] Pb2+ has the highest stability at the HBi surface, and the high geochemical affinity of Pb2+ for birnessite probably explains its billion-fold enrichment in marine ferromanganese deposits compared to seawater, which surpasses those of all other elements.[36]
Transition metal cations sorbed on vacancies were also observed to enter the underlying vacancies and become incorporated into the MnO2 layer, when their effective ionic radius[37] is close to that of Mn4+ (r = 0.53 Å). For example, Zn2+ (r = 0.74 Å) was never observed to enter a vacancy site, while Ni2+ (r = 0.69 Å) partly enters, and Co3+ (r = 0.54 Å) always does.[9][38][39] Octahedral high-spin Co2+ (r = 0.74 Å) sorbed on a vacancy is transformed into a smaller tetrahedral complex (r = 0.58 Å) to penetrate into the octahedral Mn vacancy, and is subsequently converted to the low-spin state before being oxidized to Co3+ by Mn4+, which is reduced to Mn3+.[40] The surface-catalyzed, redox-driven uptake of Co leads also to a billion-fold enrichment in marine ferromanganese deposits compared to Co between ferromanganese deposits and Pb2+.[36]
Properties and applications
[edit]In nature, photosynthetic organisms use the high oxidative ability of birnessite-type Mn4CaO5 clusters to oxidize water into molecular oxygen through the photosystem II membrane protein complex.[41]
Because of their semiconducting properties, birnessite-type materials are used in a variety of areas, including catalysis and electrochemical energy storage (batteries and pseudocapacitors). The ordering of the Mn3+ cations in triclinic birnessite, and the Mn4+ vacancies in hexagonal birnessite, both reduce the band gap,[42][43][44] and therefore enhance electrical conductivity. Half-metallic behavior was observed for MnO2 nanosheets with Mn vacancies, rendering them promising candidates for applications in spintronics.[45]
Birnessite is able to break down prions via oxidation.[46] How well this process works outside the laboratory is unclear.
-
Idealized structure for Zn-rich vernadite nanosheets precipitated in the epidermis of grass roots.
-
High-magnification transmission electron microscope (TEM) image of δ-MnO2 nano-crystals viewed parallel and perpendicular to the layer plane.
-
Structure of a cylindrically bent layer of δ-MnO2 nanosheet.
-
Structure of a spherically bent layer of δ-MnO2 nanosheet.
See also
[edit]Other manganese oxides:
References
[edit]- ^ Warr, L.N. (2021). "IMA–CNMNC approved mineral symbols". Mineralogical Magazine. 85 (3): 291–320. Bibcode:2021MinM...85..291W. doi:10.1180/mgm.2021.43. S2CID 235729616.
- ^ http://www.handbookofmineralogy.org/pdfs/birnessite.pdf Handbook of Mineralogy
- ^ http://www.mindat.org/min-680.html Mindat with location data
- ^ http://www.webmineral.com/data/Birnessite.shtml Webmineral data
- ^ Jones, L. H. P.; et al. (1956). "Birnessite, a new manganese oxide mineral from Aberdeenshire, Scotland". Mineralogical Magazine and Journal of the Mineralogical Society. 31 (235): 283–288. Bibcode:1956MinM...31..283J. doi:10.1180/minmag.1956.031.235.01.
- ^ McKeown, D. A.; et al. (2001-05-01). "Characterization of manganese oxide mineralogy in rock varnish and dendrites using X-ray absorption spectroscopy". American Mineralogist. 86 (5–6): 701–713. Bibcode:2001AmMin..86..701M. doi:10.2138/am-2001-5-611. S2CID 19647389.
- ^ Manceau, A; et al. (2003). "Molecular-Scale Speciation of Zn and Ni in Soil Ferromanganese Nodules from Loess Soils of the Mississippi Basin". Environmental Science & Technology. 37 (1): 75–80. Bibcode:2003EnST...37...75M. doi:10.1021/es025748r. PMID 12542293.
- ^ a b Isaure, M-P; et al. (2005). "Zinc mobility and speciation in soil covered by contaminated dredged sediment using micrometer-scale and bulk-averaging X-ray fluorescence, absorption and diffraction techniques". Geochimica et Cosmochimica Acta. 69 (5): 1173–1198. Bibcode:2005GeCoA..69.1173I. doi:10.1016/j.gca.2004.08.024.
- ^ a b c Manceau, A; et al. (2007). "Natural speciation of Ni, Zn, Ba, and As in ferromanganese coatings on quartz using X-ray fluorescence, absorption, and diffraction". Geochimica et Cosmochimica Acta. 71 (1): 95–128. Bibcode:2007GeCoA..71...95M. doi:10.1016/j.gca.2006.08.036.
- ^ Manceau, A.; et al. (2014). "Mineralogy and crystal chemistry of Mn, Fe, Co, Ni, and Cu in a deep-sea Pacific polymetallic nodule". American Mineralogist. 99 (10): 2068–2083. Bibcode:2014AmMin..99.2068M. doi:10.2138/am-2014-4742. S2CID 98025822.
- ^ Bodeï, S.; et al. (2007). "Formation of todorokite from vernadite in Ni-rich hemipelagic sediments". Geochimica et Cosmochimica Acta. 71 (23): 5698–5716. Bibcode:2007GeCoA..71.5698B. doi:10.1016/j.gca.2007.07.020.
- ^ Bargar, J.R.; et al. (2009). "Structural characterization of terrestrial microbial Mn oxides from Pinal Creek, AZ". Geochimica et Cosmochimica Acta. 73 (4): 889–910. Bibcode:2009GeCoA..73..889B. doi:10.1016/j.gca.2008.10.036. S2CID 59065579.
- ^ Tebo, B. M.; et al. (2004-05-19). "Biogenic manganese oxides: Properties and mechanisms of formation". Annual Review of Earth and Planetary Sciences. 32: 287–328. Bibcode:2004AREPS..32..287T. doi:10.1146/annurev.earth.32.101802.120213.
- ^ a b Lanson, B; et al. (2002). "Structure of synthetic Na-birnessite: Evidence for a triclinic one-layer unit cell". American Mineralogist. 87 (11–12): 1662–1671. Bibcode:2002AmMin..87.1662L. doi:10.2138/am-2002-11-1215. S2CID 53443294.
- ^ a b Drits, V. A.; et al. (1997). "Structure of synthetic monoclinic Na-rich birnessite and hexagonal birnessite; I, Results from X-ray diffraction and selected-area electron diffraction". American Mineralogist. 82 (9–10): 946–961. Bibcode:1997AmMin..82..946D. doi:10.2138/am-1997-9-1012. S2CID 56030552.
- ^ a b Silvester, E.; et al. (1997). "Structure of synthetic monoclinic Na-rich birnessite and hexagonal birnessite; II, Results from chemical studies and EXAFS spectroscopy". American Mineralogist. 82 (9–10): 962–978. Bibcode:1997AmMin..82..962S. doi:10.2138/am-1997-9-1013. S2CID 55969753.
- ^ a b Lanson, B; et al. (2000). "Structure of H-exchanged hexagonal birnessite and its mechanism of formation from Na-rich monoclinic buserite at low pH". American Mineralogist. 85 (5–6): 826–838. Bibcode:2000AmMin..85..826L. doi:10.2138/am-2000-5-625. S2CID 55586944.
- ^ Manceau, A; et al. (2013-02-01). "Short-range and long-range order of phyllomanganate nanoparticles determined using high-energy X-ray scattering". Journal of Applied Crystallography. 46: 193–209. doi:10.1107/S0021889812047917. S2CID 56356250.
- ^ Giovanoli, R. (1980). "Vernadite is random-stacked birnessite". Mineralium Deposita. 15 (2): 251–253. Bibcode:1980MinDe..15..251G. doi:10.1007/bf00206520. S2CID 104363781.
- ^ Manceau, A.; et al. (1988). "Structure of Mn and Fe oxides and oxyhydroxides: A topological approach by EXAFS". Physics and Chemistry of Minerals. 15 (3): 283–295. Bibcode:1988PCM....15..283M. doi:10.1007/bf00307518. S2CID 97465847.
- ^ Hochella, M. F. (2005). "Environmentally important, poorly crystalline Fe/Mn hydrous oxides: Ferrihydrite and a possibly new vernadite-like mineral from the Clark Fork River Superfund Complex". American Mineralogist. 90 (4): 718–724. Bibcode:2005AmMin..90..718H. doi:10.2138/am.2005.1591. S2CID 54664549.
- ^ Lee, S; et al. (2019). "The structure and crystal chemistry of vernadite in ferromanganese crusts". Acta Crystallographica Section B. 75 (4): 591–598. doi:10.1107/S2052520619006528. OSTI 1559941. PMID 32830716. S2CID 202084338.
- ^ Lanson, B; et al. (2002). "Structure of heavy-metal sorbed birnessite: Part 1. Results from X-ray diffraction". American Mineralogist. 87 (11–12): 1631–1645. Bibcode:2002AmMin..87.1631L. doi:10.2138/am-2002-11-1213. S2CID 52255943.
- ^ Drits, V. A.; et al. (2002). "Structure of heavy-metal sorbed birnessite: Part 2. Results from electron diffraction". American Mineralogist. 87 (11–12): 1646–1661. Bibcode:2002AmMin..87.1646D. doi:10.2138/am-2002-11-1214. S2CID 52241298.
- ^ Manceau, A; et al. (2002). "Structure of heavy metal sorbed birnessite. Part III: Results from powder and polarized extended X-ray absorption fine structure spectroscopy". Geochimica et Cosmochimica Acta. 66 (15): 2639–2663. Bibcode:2002GeCoA..66.2639M. doi:10.1016/s0016-7037(02)00869-4.
- ^ Marcus, M. A; et al. (2004). "Mn, Fe, Zn and As speciation in a fast-growing ferromanganese marine nodule". Geochimica et Cosmochimica Acta. 68 (14): 3125–3136. Bibcode:2004GeCoA..68.3125M. doi:10.1016/j.gca.2004.01.015. S2CID 33694906.
- ^ Takahashi, Y; et al. (2007). "Chemical and structural control of the partitioning of Co, Ce, and Pb in marine ferromanganese oxides". Geochimica et Cosmochimica Acta. 71 (4): 984–1008. Bibcode:2007GeCoA..71..984T. doi:10.1016/j.gca.2006.11.016.
- ^ Lanson, B; et al. (2008). "Formation of Zn–Ca phyllomanganate nanoparticles in grass roots". Geochimica et Cosmochimica Acta. 72 (10): 2478–2490. Bibcode:2008GeCoA..72.2478L. doi:10.1016/j.gca.2008.02.022. S2CID 16075545.
- ^ Wick, S; et al. (2019). "Thallium Sorption onto Manganese Oxides". Environmental Science & Technology. 53 (22): 13168–13178. Bibcode:2019EnST...5313168W. doi:10.1021/acs.est.9b04454. hdl:20.500.11850/383584. PMID 31674774. S2CID 207815275.
- ^ Manceau, A.; et al. (2022). "Crystal Chemistry of Thallium in Marine Ferromanganese Deposits". ACS Earth and Space Chemistry. 6 (5): 1269–1285. Bibcode:2022ESC.....6.1269M. doi:10.1021/acsearthspacechem.1c00447.
- ^ Wang, Y; et al. (2012). "Sorption behavior of heavy metals on birnessite: Relationship with its Mn average oxidation state and implications for types of sorption sites". Chemical Geology. 292–293: 25–34. Bibcode:2012ChGeo.292...25W. doi:10.1016/j.chemgeo.2011.11.001.
- ^ Murray, J. W. (1975). "The interaction of metal ions at the manganese dioxide-solution interface". Geochimica et Cosmochimica Acta. 39 (4): 505–519. Bibcode:1975GeCoA..39..505M. doi:10.1016/0016-7037(75)90103-9.
- ^ Appelo, C.A.J.; et al. (1999). "A consistent model for surface complexation on birnessite (δ-MnO2) and its application to a column experiment". Geochimica et Cosmochimica Acta. 63 (19–20): 3039–3048. Bibcode:1999GeCoA..63.3039A. doi:10.1016/s0016-7037(99)00231-8.
- ^ Kwon, K. D.; et al. (2013). "Understanding the trends in transition metal sorption by vacancy sites in birnessite". Geochimica et Cosmochimica Acta. 101: 222–232. Bibcode:2013GeCoA.101..222K. doi:10.1016/j.gca.2012.08.038.
- ^ Manceau, A; et al. (2021). "Nature of High- and Low-Affinity Metal Surface Sites on Birnessite Nanosheets". ACS Earth and Space Chemistry. 5 (1): 66–76. Bibcode:2021ESC.....5...66M. doi:10.1021/acsearthspacechem.0c00278. S2CID 234304816.
- ^ a b Hein, J.R.; et al. (2014), "Deep-Ocean Ferromanganese Crusts and Nodules", Treatise on Geochemistry, Elsevier, pp. 273–291, doi:10.1016/b978-0-08-095975-7.01111-6, ISBN 9780080983004, retrieved 2021-09-26
- ^ Shannon, R. D. (1976). "Revised effective ionic radii and systematic studies of interatomic distances in halides and chalcogenides". Acta Crystallographica Section A. 32 (5): 751–767. Bibcode:1976AcCrA..32..751S. doi:10.1107/s0567739476001551.
- ^ Manceau, A; et al. (1997). "Structural mechanism of Co2+ oxidation by the phyllomanganate buserite". American Mineralogist. 82 (11–12): 1150–1175. Bibcode:1997AmMin..82.1150M. doi:10.2138/am-1997-11-1213. S2CID 54923713.
- ^ Peacock, C. L.; et al. (2007). "Crystal-chemistry of Ni in marine ferromanganese crusts and nodules". American Mineralogist. 92 (7): 1087–1092. Bibcode:2007AmMin..92.1087P. doi:10.2138/am.2007.2378. S2CID 98074547.
- ^ Manceau, A.; Steinmann, S. (2022). "Density Functional Theory Modeling of the Oxidation Mechanism of Co(II) by Birnessite". ACS Earth and Space Chemistry. 6 (8): 2063–2075. Bibcode:2022ESC.....6.2063M. doi:10.1021/acsearthspacechem.2c00122. S2CID 251086409.
- ^ Chernev, P.; et al. (2021). "Publisher Correction: Light-driven formation of manganese oxide by today's photosystem II supports evolutionarily ancient manganese-oxidizing photosynthesis". Nature Communications. 12 (1): 419. doi:10.1038/s41467-020-20868-9. PMC 7804171. PMID 33436628.
- ^ Lucht, K. P.; et al. (2015). "Birnessite: A Layered Manganese Oxide To Capture Sunlight for Water-Splitting Catalysis". The Journal of Physical Chemistry C. 119 (40): 22838–22846. doi:10.1021/acs.jpcc.5b07860.
- ^ Kwon, K. D.; et al. (2008). "Defect-Induced Photoconductivity in Layered Manganese Oxides: A Density Functional Theory Study". Physical Review Letters. 100 (14): 146601. Bibcode:2008PhRvL.100n6601K. doi:10.1103/physrevlett.100.146601. PMID 18518059. S2CID 2146794.
- ^ Gao, P.; et al. (2020). "The Role of Cation Vacancies in Electrode Materials for Enhanced Electrochemical Energy Storage: Synthesis, Advanced Characterization, and Fundamentals". Advanced Energy Materials. 10 (14): 1903780. doi:10.1002/aenm.201903780. S2CID 214006754.
- ^ Wang, H; et al. (2014). "Half-Metallicity in Single-Layered Manganese Dioxide Nanosheets by Defect Engineering". Angewandte Chemie. 127 (4): 1211–1215. doi:10.1002/ange.201410031.
- ^ Russo, F.; et al. (2009). "Pathogenic prion protein is degraded by a manganese oxide mineral found in soils". Journal of General Virology. 90 (Pt 1): 275–280. doi:10.1099/vir.0.003251-0. PMID 19088299.