1-Octen-3-ol: Difference between revisions
No edit summary |
m rmv duplicate parm |
||
| (12 intermediate revisions by 9 users not shown) | |||
| Line 1: | Line 1: | ||
{{cs1 config|name-list-style=vanc}} |
|||
{{Redirect-distinguish|Octenol|octanal|octanol|Octonal (disambiguation){{!}}octonal}} |
{{Redirect-distinguish|Octenol|octanal|octanol|Octonal (disambiguation){{!}}octonal}} |
||
{{chembox |
{{chembox |
||
| Line 6: | Line 7: | ||
| ImageSize=200px |
| ImageSize=200px |
||
| PIN=Oct-1-en-3-ol |
| PIN=Oct-1-en-3-ol |
||
| OtherNames=Amyl vinyl carbinol; 1-Vinylhexanol; Matsutake alcohol; Vinyl amyl carbinol; Vinyl hexanol; Matsuica alcohol; Mushroom alcohol; 3-Hydroxy-1-octene |
| OtherNames=Amyl vinyl carbinol; 1-Vinylhexanol; Matsutake alcohol; Vinyl amyl carbinol; Vinyl hexanol; Matsuica alcohol; Mushroom alcohol; 3-Hydroxy-1-octene; Octenol |
||
|Section1={{Chembox Identifiers |
|Section1={{Chembox Identifiers |
||
| ChemSpiderID_Ref = {{chemspidercite|correct|chemspider}} |
| ChemSpiderID_Ref = {{chemspidercite|correct|chemspider}} |
||
| ChEBI_Ref = {{ebicite|correct|EBI}} |
| ChEBI_Ref = {{ebicite|correct|EBI}} |
||
| ChEBI = 34118 |
| ChEBI = 34118 |
||
| ChEBI1 = 39932 |
|||
| ChEBI2 = 46735 |
|||
| ChEMBL = 3183573 |
|||
| ChEMBL1 = 1230177 |
|||
| ChemSpiderID = 17778 |
| ChemSpiderID = 17778 |
||
| ChemSpiderID1 = : 5360388 |
|||
| ChemSpiderID2 = : 2007013 |
|||
| DrugBank2 = DB03025 |
|||
| EC_number = 222-226-0 |
|||
| Gmelin = 648361 |
|||
| KEGG_Ref = {{keggcite|correct|kegg}} |
| KEGG_Ref = {{keggcite|correct|kegg}} |
||
| KEGG = C14272 |
| KEGG = C14272 |
||
| InChI = 1/C8H16O/c1-3-5-6-7-8(9)4-2/h4,8-9H,2-3,5-7H2,1H3 |
| InChI = 1/C8H16O/c1-3-5-6-7-8(9)4-2/h4,8-9H,2-3,5-7H2,1H3 |
||
| ⚫ | |||
| InChIKey = VSMOENVRRABVKN-UHFFFAOYAB |
| InChIKey = VSMOENVRRABVKN-UHFFFAOYAB |
||
| InChIKey1 =VSMOENVRRABVKN-QMMMGPOBSA-N |
|||
| InChIKey2 =VSMOENVRRABVKN-MRVPVSSYSA-N |
|||
| StdInChI_Ref = {{stdinchicite|correct|chemspider}} |
| StdInChI_Ref = {{stdinchicite|correct|chemspider}} |
||
| StdInChI = 1S/C8H16O/c1-3-5-6-7-8(9)4-2/h4,8-9H,2-3,5-7H2,1H3 |
| StdInChI = 1S/C8H16O/c1-3-5-6-7-8(9)4-2/h4,8-9H,2-3,5-7H2,1H3 |
||
| Line 25: | Line 36: | ||
| CASNo1_Ref = {{cascite|correct|??}} |
| CASNo1_Ref = {{cascite|correct|??}} |
||
| CASNo1 = 3687-48-7 |
| CASNo1 = 3687-48-7 |
||
| |
| index1_label = (''R'')-(−) |
||
| |
| index2_label = (''S'')-(+) |
||
| CASNo2_Ref = {{cascite|correct|??}} |
| CASNo2_Ref = {{cascite|correct|??}} |
||
| CASNo2 = 24587-53-9 |
| CASNo2 = 24587-53-9 |
||
| PubChem=18827 |
| PubChem=18827 |
||
| PubChem1 = 6992244 |
|||
| UNII_Ref = {{fdacite|correct|FDA}} |
| UNII_Ref = {{fdacite|correct|FDA}} |
||
| UNII = WXB511GE38 |
| UNII = WXB511GE38 |
||
| UNII1_Ref = {{fdacite|correct|FDA}} |
| UNII1_Ref = {{fdacite|correct|FDA}} |
||
| UNII1 = BYV0MEV7V1 |
| UNII1 = BYV0MEV7V1 |
||
| UNII1_Comment = (''R'')-(–) |
|||
| UNII2_Ref = {{fdacite|correct|FDA}} |
| UNII2_Ref = {{fdacite|correct|FDA}} |
||
| UNII2 = 07D31239FH |
| UNII2 = 07D31239FH |
||
| UNII2_Comment = (''S'')-(+) |
|||
| SMILES=CCCCCC(C=C)O |
| SMILES=CCCCCC(C=C)O |
||
| ⚫ | |||
| SMILES2 = CCCCC[C@H](O)C=C |
|||
}} |
}} |
||
|Section2={{Chembox Properties |
|Section2={{Chembox Properties |
||
| C=8 | H=16 | O=1 |
| C=8 | H=16 | O=1 |
||
| Appearance= |
| Appearance= |
||
| Density= |
| Density= 0.837 g/mL |
||
| MeltingPt= |
| MeltingPt= |
||
| BoilingPt= |
| BoilingPt= 174 ºC at 1 atm |
||
| VaporPressure = 0.3 kPa (at 50 °C) |
|||
| Solubility= |
| Solubility= |
||
}} |
}} |
||
|Section3={{Chembox Hazards |
|Section3={{Chembox Hazards |
||
| MainHazards= |
| MainHazards= |
||
| FlashPt= |
| FlashPt= 68 ºC |
||
| AutoignitionPt = |
| AutoignitionPt = 245 ºC |
||
| ExploLimits = 0.9% (low) to 8% (high) |
|||
|GHSPictograms = {{GHS07}} |
|||
|GHSSignalWord = Warning |
|||
|NFPA-H = 2 |
|||
|NFPA-F = 2 |
|||
|NFPA-R = 0 |
|||
|LD50 = 340 mg/kg (rat) |
|||
|ExternalSDS = [https://www.fishersci.com/store/msds?partNumber=AC129470050&productDescription=1-OCTEN-3-OL%2C+98%25+5GR&vendorId=VN00032119&countryCode=US&language=en Fisher Scientific] |
|||
}} |
}} |
||
}} |
}} |
||
'''1-Octen-3-ol''', '''octenol''' for short and also known as '''mushroom alcohol''',<ref>{{ |
'''1-Octen-3-ol''', '''octenol''' for short and also known as '''mushroom alcohol''',<ref>{{cite web |
||
|title = 1-Octen-3-ol, Mushroom alcohol |
|title = 1-Octen-3-ol, Mushroom alcohol |
||
|access-date = 2019-08-29 |
|access-date = 2019-08-29 |
||
|url = http://www.hmdb.ca/metabolites/HMDB0031299 |
|url = http://www.hmdb.ca/metabolites/HMDB0031299 |
||
}}</ref> is a chemical that attracts biting insects such as [[mosquito]]es. It is contained in human breath and sweat, and it is believed that [[insect repellent]] [[DEET]] works by blocking the insects' octenol [[odorant]] [[receptor (biochemistry)|receptor]]s.<ref name=petherick>{{cite journal |first=Anna |last=Petherick |
}}</ref> is a chemical that attracts biting insects such as [[mosquito]]es. It is contained in human breath and sweat, and it is believed that [[insect repellent]] [[DEET]] works by blocking the insects' octenol [[odorant]] [[receptor (biochemistry)|receptor]]s.<ref name=petherick>{{cite journal |first=Anna |last=Petherick |title=How DEET jams insects' smell sensors |journal=Nature News |date=2008-03-13 |url= http://www.nature.com/news/2008/080313/full/news.2008.672.html |archive-url=https://web.archive.org/web/20080315162356/http://www.nature.com/news/2008/080313/full/news.2008.672.html | archive-date=15 March 2008 |url-status=live |doi=10.1038/news.2008.672 |doi-access=free }}</ref><ref>{{cite journal | vauthors = Ditzen M, Pellegrino M, Vosshall LB | title = Insect odorant receptors are molecular targets of the insect repellent DEET | journal = Science | volume = 319 | issue = 5871 | pages = 1838–42 | date = March 2008 | pmid = 18339904 | doi = 10.1126/science.1153121 | bibcode = 2008Sci...319.1838D | s2cid = 18499590 | author3-link = Leslie B. Vosshall }}</ref><ref>{{cite journal | vauthors = Syed Z, Leal WS | title = Mosquitoes smell and avoid the insect repellent DEET | journal = Proceedings of the National Academy of Sciences of the United States of America | volume = 105 | issue = 36 | pages = 13598–603 | date = September 2008 | pmid = 18711137 | pmc = 2518096 | doi = 10.1073/pnas.0805312105 | doi-access = free }}</ref> |
||
| ⚫ | The name “mushroom Alcohol” for 1-octen-3-ol comes from it first isolation by Murahashi in 1936 and 1938 from crushed matsutake mushrooms. <ref>{{cite journal | last1 = Murahashi | first1 = S. | title = Sci. Pap. Inst. Phys. Chem. Res. (Jpn.) 34, 155 | journal = Chemical Absracts | volume = 31 | page = 21617 }}</ref><ref>{{cite journal | last1 = Murahashi | first1 = S. | title = Sci. Pap. Inst. Phys. Chem. Res. (Jpn.) 30, 263 | journal = Chemical Absracts | volume = 32 | page = 27078 }}</ref>A recent study on volatiles of this mushroom has shown this compound is only produced upon tissue disruption.<ref>{{cite journal |journal= Biochemical Systematics and Ecology |volume=35 | year=2007 |title= Changing volatile compounds from mycelium and sporocarp of American matsutake mushroom, |
||
| ⚫ | This alcohol is found in many other mushrooms where it may play a role as an antifeedant.<ref>{{cite journal | last1 = Wood | first1 = William F. | last2 = Archer | first2 = Cynthia L. | last3 = Largent | first3 = David L. | year = 2001 | title = 1-Octen-3-ol, a banana slug antifeedant from mushrooms | journal = Biochemical Systematics and Ecology | volume = 29 | pages = 531–533| doi=10.1016/s0305-1978(00)00076-4}}</ref> |
||
| ⚫ | The name “mushroom Alcohol” for 1-octen-3-ol comes from it first isolation by Murahashi in 1936 and 1938 from crushed matsutake mushrooms. <ref>{{cite journal | last1 = Murahashi | first1 = S. | title = Sci. Pap. Inst. Phys. Chem. Res. (Jpn.) 34, 155 | journal = Chemical Absracts | volume = 31 | page = 21617 }}</ref><ref>{{cite journal | last1 = Murahashi | first1 = S. | title = Sci. Pap. Inst. Phys. Chem. Res. (Jpn.) 30, 263 | journal = Chemical Absracts | volume = 32 | page = 27078 }}</ref>A recent study on volatiles of this mushroom has shown this compound is only produced upon tissue disruption.<ref>{{cite journal |journal= Biochemical Systematics and Ecology |volume=35 | year=2007 |title= Changing volatile compounds from mycelium and sporocarp of American matsutake mushroom, ''Tricholoma magnivelare'' |author1=Wood W. F. |author2=Lefevre C. K. |issue=9 | doi= 10.1016/j.bse.2007.03.001 |pages=634–636|bibcode=2007BioSE..35..634W }}</ref> |
||
| ⚫ | This alcohol is found in many other mushrooms where it may play a role as an [[antifeedant]].<ref>{{cite journal | last1 = Wood | first1 = William F. | last2 = Archer | first2 = Cynthia L. | last3 = Largent | first3 = David L. | year = 2001 | title = 1-Octen-3-ol, a banana slug antifeedant from mushrooms | journal = Biochemical Systematics and Ecology | volume = 29 | issue = 5 | pages = 531–533| doi=10.1016/s0305-1978(00)00076-4| pmid = 11274773 | bibcode = 2001BioSE..29..531W }}</ref> |
||
== Natural occurrence== |
== Natural occurrence== |
||
| Line 77: | Line 93: | ||
|archive-date = 2009-04-27 |
|archive-date = 2009-04-27 |
||
|url-status = dead |
|url-status = dead |
||
}}</ref> Octenol is responsible for the moldy odor of damp indoor environments.<ref>{{cite journal | vauthors = Bennett JW, Inamdar AA | title = Are Some Fungal Volatile Organic Compounds (VOCs) Mycotoxins? | journal = Toxins | year = 2015 | volume = 7 | issue = 9 | pages = 3785–3804 | doi = 10.3390/toxins7093785 | doi-access = free | pmc = 4591661 |
|||
}}</ref> |
|||
| pmid = 26402705 }}</ref> |
|||
It is also a [[wine fault]], defined as a [[cork taint]], occurring in wines made with [[Botrytis cinerea|bunch rot]] contaminated grape.<ref name="pmid23675852">{{cite journal | vauthors = Steel CC, Blackman JW, Schmidtke LM | title = Grapevine bunch rots: |
It is also a [[wine fault]], defined as a [[cork taint]], occurring in wines made with [[Botrytis cinerea|bunch rot]] contaminated grape.<ref name="pmid23675852">{{cite journal | vauthors = Steel CC, Blackman JW, Schmidtke LM | title = Grapevine bunch rots: Impacts on wine composition, quality, and potential procedures for the removal of wine faults | journal = Journal of Agricultural and Food Chemistry | volume = 61 | issue = 22 | pages = 5189–206 | date = June 2013 | pmid = 23675852 | doi = 10.1021/jf400641r }}</ref> |
||
==Properties== |
==Properties== |
||
1- |
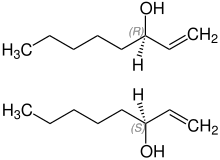
1-octen-3-ol is a [[Alcohol (chemistry)#Systematic names|secondary alcohol]] derived from [[1-octene]]. It exists in the form of two [[enantiomer]]s, (''R'')-(−)-1-octen-3-ol and (''S'')-(+)-1-octen-3-ol. |
||
:[[File:(RS)-1-Octen-3-ol FormulaV1.svg|thumb|left|The two enantiomers of 1-octen-3-ol]]{{clear-left}} |
:[[File:(RS)-1-Octen-3-ol FormulaV1.svg|thumb|left|The two enantiomers of 1-octen-3-ol]]{{clear-left}} |
||
== Synthesis == |
== Synthesis == |
||
Two possible lab syntheses of 1-octen-3-ol are:<ref>{{ |
Two possible lab syntheses of 1-octen-3-ol are:<ref>{{cite journal|last1=Wnuk|first1=S.|last2=Kinastowski|first2=S.|last3=Kamiński|first3=E.|date=1983|title=Synthesis and analysis of 1-octen-3-ol, the main flavour component of mushrooms|url=https://pubmed.ncbi.nlm.nih.gov/6684212/|journal=Die Nahrung|volume=27|issue=5|pages=479–486|doi=10.1002/food.19830270523|issn=0027-769X|pmid=6684212}}</ref> |
||
* by the Grignard reaction of [[acrolein]] and amyl iodide |
* by the Grignard reaction of [[acrolein]] and amyl iodide |
||
* by the selective reduction of [[Oct-1-en-3-one|1-octen-3-one]] |
* by the selective reduction of [[Oct-1-en-3-one|1-octen-3-one]] |
||
Biochemically, 1-octen-3-ol is generated from the [[Lipid peroxidation|peroxidation]] of [[linoleic acid]], catalyzed by a [[lipoxygenase]], followed by cleavage of the resulting hydroperoxide with the help of a [[hydroperoxide lyase]]. This reaction takes place in cheese and is used in biotechnology to produce the ''(R)''-isomer.<ref>{{ |
Biochemically, 1-octen-3-ol is generated from the [[Lipid peroxidation|peroxidation]] of [[linoleic acid]], catalyzed by a [[lipoxygenase]], followed by cleavage of the resulting hydroperoxide with the help of a [[hydroperoxide lyase]]. This reaction takes place in cheese and is used in biotechnology to produce the ''(R)''-isomer.<ref>{{cite journal|last1=Matsui|first1=Kenji|last2=Sasahara|first2=Satomi|last3=Akakabe|first3=Yoshihiko|last4=Kajiwara|first4=Tadahiko|date=2003|title=Linoleic acid 10-hydroperoxide as an intermediate during formation of 1-octen-3-ol from linoleic acid in Lentinus decadetes|journal=Bioscience, Biotechnology, and Biochemistry|volume=67|issue=10|pages=2280–2282|doi=10.1271/bbb.67.2280|issn=0916-8451|pmid=14586122|s2cid=46173472 |doi-access=free}}</ref><ref>{{cite book |last1=Min Kuo |first1=Tsung |url=https://www.worldcat.org/oclc/48691412 |title=Lipid biotechnology |last2=Gardner |first2=Harold W. |date=2002 |publisher=Marcel Dekker |isbn=0-585-40371-6 |location=New York |oclc=48691412}}</ref> |
||
[[File:Octenol-biosynthesis.png|thumb|'''Biosynthesis of (''R'')-1-octen-3-ol''': '''1''') linoleic acid, '''2''') (8''E'',12''Z'')-10-hydroperoxyoctadecadienoic acid, '''3''') (''R'')-1-octen-3-ol, '''4''') (8''E'')-10-oxodecenoic acid, '''5''') lipoxygenase, '''6''') hydroperoxide lyase.|center|399x399px]] |
[[File:Octenol-biosynthesis.png|thumb|'''Biosynthesis of (''R'')-1-octen-3-ol''': '''1''') linoleic acid, '''2''') (8''E'',12''Z'')-10-hydroperoxyoctadecadienoic acid, '''3''') (''R'')-1-octen-3-ol, '''4''') (8''E'')-10-oxodecenoic acid, '''5''') lipoxygenase, '''6''') hydroperoxide lyase.|center|399x399px]] |
||
| Line 98: | Line 115: | ||
Octenol is used, sometimes in combination with [[carbon dioxide]], to attract insects in order to kill them with certain electrical devices.<ref name="epa">{{cite web |date=2007-07-05 |title=Biopesticides Fact Sheet for Octenol |url=https://www3.epa.gov/pesticides/chem_search/reg_actions/registration/fs_G-126_05-Jul-07.pdf |access-date=2022-06-28 |work=EPA fact sheet}}</ref> |
Octenol is used, sometimes in combination with [[carbon dioxide]], to attract insects in order to kill them with certain electrical devices.<ref name="epa">{{cite web |date=2007-07-05 |title=Biopesticides Fact Sheet for Octenol |url=https://www3.epa.gov/pesticides/chem_search/reg_actions/registration/fs_G-126_05-Jul-07.pdf |access-date=2022-06-28 |work=EPA fact sheet}}</ref> |
||
The name 'mushroom alcohol' is used because octenol is the main flavor component of [[mushroom]]s.<ref>{{ |
The name 'mushroom alcohol' is used because octenol is the main flavor component of [[mushroom]]s.<ref>{{cite web|url=http://www.thegoodscentscompany.com/data/rw1024051.html|title=1-octen-3-ol|date=|website=thegoodscentscompany.com|access-date=2015-05-31}}</ref> |
||
==Health and safety== |
==Health and safety== |
||
Octenol is approved by the [[Food and Drug Administration (United States)|U.S. Food and Drug Administration]] as a [[food additive]].<ref>{{ |
Octenol is approved by the [[Food and Drug Administration (United States)|U.S. Food and Drug Administration]] as a [[food additive]].<ref>{{cite web |
||
|author = US FDAs Center for Food Safety and Applied Nutrition |
|author = US FDAs Center for Food Safety and Applied Nutrition |
||
|title = US FDA/CFSAN – EAFUS List |
|title = US FDA/CFSAN – EAFUS List |
||
| Line 115: | Line 132: | ||
== See also == |
== See also == |
||
* [[Olfactory receptor]] |
* [[Olfactory receptor]] |
||
* [[Oct-1-en-3-one]], the ketone analog that gives blood on skin its typical metallic, mushroom-like smell<ref>{{cite journal | vauthors = Glindemann D, Dietrich A, Staerk HJ, Kuschk P | title = The two odors of iron when touched or pickled: (skin) carbonyl compounds and organophosphines | journal = Angewandte Chemie | volume = 45 | issue = 42 | pages = 7006–9 | date = October 2006 | pmid = 17009284 | doi = 10.1002/anie.200602100 | doi-access = |
* [[Oct-1-en-3-one]], the ketone analog that gives blood on skin its typical metallic, mushroom-like smell<ref>{{cite journal | vauthors = Glindemann D, Dietrich A, Staerk HJ, Kuschk P | title = The two odors of iron when touched or pickled: (skin) carbonyl compounds and organophosphines | journal = Angewandte Chemie | volume = 45 | issue = 42 | pages = 7006–9 | date = October 2006 | pmid = 17009284 | doi = 10.1002/anie.200602100 | doi-access = | s2cid = 45055136 }} |
||
</ref> |
</ref> |
||
* [[1-Octen-3-yl acetate]], the acetate ester of this compound |
* [[1-Octen-3-yl acetate]], the acetate ester of this compound |
||
Latest revision as of 16:17, 17 August 2024

| |
| Names | |
|---|---|
| Preferred IUPAC name
Oct-1-en-3-ol | |
| Other names
Amyl vinyl carbinol; 1-Vinylhexanol; Matsutake alcohol; Vinyl amyl carbinol; Vinyl hexanol; Matsuica alcohol; Mushroom alcohol; 3-Hydroxy-1-octene; Octenol
| |
| Identifiers | |
| |
3D model (JSmol)
|
|
| ChEBI |
|
| ChEMBL |
|
| ChemSpider |
|
| DrugBank |
|
| ECHA InfoCard | 100.020.206 |
| EC Number |
|
| 648361 | |
| KEGG | |
PubChem CID
|
|
| UNII |
|
CompTox Dashboard (EPA)
|
|
| |
| |
| Properties | |
| C8H16O | |
| Molar mass | 128.215 g·mol−1 |
| Density | 0.837 g/mL |
| Boiling point | 174 ºC at 1 atm |
| Vapor pressure | 0.3 kPa (at 50 °C) |
| Hazards | |
| GHS labelling: | |

| |
| Warning | |
| NFPA 704 (fire diamond) | |
| Flash point | 68 ºC |
| 245 ºC | |
| Explosive limits | 0.9% (low) to 8% (high) |
| Lethal dose or concentration (LD, LC): | |
LD50 (median dose)
|
340 mg/kg (rat) |
| Safety data sheet (SDS) | Fisher Scientific |
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa).
| |
1-Octen-3-ol, octenol for short and also known as mushroom alcohol,[1] is a chemical that attracts biting insects such as mosquitoes. It is contained in human breath and sweat, and it is believed that insect repellent DEET works by blocking the insects' octenol odorant receptors.[2][3][4]
The name “mushroom Alcohol” for 1-octen-3-ol comes from it first isolation by Murahashi in 1936 and 1938 from crushed matsutake mushrooms. [5][6]A recent study on volatiles of this mushroom has shown this compound is only produced upon tissue disruption.[7] This alcohol is found in many other mushrooms where it may play a role as an antifeedant.[8]
Natural occurrence
[edit]Octenol is produced by several plants and fungi, including edible mushrooms and lemon balm. Octenol is formed during oxidative breakdown of linoleic acid.[9] Octenol is responsible for the moldy odor of damp indoor environments.[10]
It is also a wine fault, defined as a cork taint, occurring in wines made with bunch rot contaminated grape.[11]
Properties
[edit]1-octen-3-ol is a secondary alcohol derived from 1-octene. It exists in the form of two enantiomers, (R)-(−)-1-octen-3-ol and (S)-(+)-1-octen-3-ol.
Synthesis
[edit]Two possible lab syntheses of 1-octen-3-ol are:[12]
- by the Grignard reaction of acrolein and amyl iodide
- by the selective reduction of 1-octen-3-one
Biochemically, 1-octen-3-ol is generated from the peroxidation of linoleic acid, catalyzed by a lipoxygenase, followed by cleavage of the resulting hydroperoxide with the help of a hydroperoxide lyase. This reaction takes place in cheese and is used in biotechnology to produce the (R)-isomer.[13][14]

Uses
[edit]Octenol is used, sometimes in combination with carbon dioxide, to attract insects in order to kill them with certain electrical devices.[15]
The name 'mushroom alcohol' is used because octenol is the main flavor component of mushrooms.[16]
Health and safety
[edit]Octenol is approved by the U.S. Food and Drug Administration as a food additive.[17] It is of moderate toxicity with an LD50 of 340 mg/kg.[15]
In an animal study, octenol has been found to disrupt dopamine homeostasis and may be an environmental agent involved in parkinsonism.[18]
See also
[edit]- Olfactory receptor
- Oct-1-en-3-one, the ketone analog that gives blood on skin its typical metallic, mushroom-like smell[19]
- 1-Octen-3-yl acetate, the acetate ester of this compound
References
[edit]- ^ "1-Octen-3-ol, Mushroom alcohol". Retrieved 2019-08-29.
- ^ Petherick A (2008-03-13). "How DEET jams insects' smell sensors". Nature News. doi:10.1038/news.2008.672. Archived from the original on 15 March 2008.
- ^ Ditzen M, Pellegrino M, Vosshall LB (March 2008). "Insect odorant receptors are molecular targets of the insect repellent DEET". Science. 319 (5871): 1838–42. Bibcode:2008Sci...319.1838D. doi:10.1126/science.1153121. PMID 18339904. S2CID 18499590.
- ^ Syed Z, Leal WS (September 2008). "Mosquitoes smell and avoid the insect repellent DEET". Proceedings of the National Academy of Sciences of the United States of America. 105 (36): 13598–603. doi:10.1073/pnas.0805312105. PMC 2518096. PMID 18711137.
- ^ Murahashi S. "Sci. Pap. Inst. Phys. Chem. Res. (Jpn.) 34, 155". Chemical Absracts. 31: 21617.
- ^ Murahashi S. "Sci. Pap. Inst. Phys. Chem. Res. (Jpn.) 30, 263". Chemical Absracts. 32: 27078.
- ^ Wood W. F., Lefevre C. K. (2007). "Changing volatile compounds from mycelium and sporocarp of American matsutake mushroom, Tricholoma magnivelare". Biochemical Systematics and Ecology. 35 (9): 634–636. Bibcode:2007BioSE..35..634W. doi:10.1016/j.bse.2007.03.001.
- ^ Wood WF, Archer CL, Largent DL (2001). "1-Octen-3-ol, a banana slug antifeedant from mushrooms". Biochemical Systematics and Ecology. 29 (5): 531–533. Bibcode:2001BioSE..29..531W. doi:10.1016/s0305-1978(00)00076-4. PMID 11274773.
- ^ "Chemical properties of attractants". Archived from the original on 2009-04-27. Retrieved 2010-06-08.
- ^ Bennett JW, Inamdar AA (2015). "Are Some Fungal Volatile Organic Compounds (VOCs) Mycotoxins?". Toxins. 7 (9): 3785–3804. doi:10.3390/toxins7093785. PMC 4591661. PMID 26402705.
- ^ Steel CC, Blackman JW, Schmidtke LM (June 2013). "Grapevine bunch rots: Impacts on wine composition, quality, and potential procedures for the removal of wine faults". Journal of Agricultural and Food Chemistry. 61 (22): 5189–206. doi:10.1021/jf400641r. PMID 23675852.
- ^ Wnuk S, Kinastowski S, Kamiński E (1983). "Synthesis and analysis of 1-octen-3-ol, the main flavour component of mushrooms". Die Nahrung. 27 (5): 479–486. doi:10.1002/food.19830270523. ISSN 0027-769X. PMID 6684212.
- ^ Matsui K, Sasahara S, Akakabe Y, Kajiwara T (2003). "Linoleic acid 10-hydroperoxide as an intermediate during formation of 1-octen-3-ol from linoleic acid in Lentinus decadetes". Bioscience, Biotechnology, and Biochemistry. 67 (10): 2280–2282. doi:10.1271/bbb.67.2280. ISSN 0916-8451. PMID 14586122. S2CID 46173472.
- ^ Min Kuo T, Gardner HW (2002). Lipid biotechnology. New York: Marcel Dekker. ISBN 0-585-40371-6. OCLC 48691412.
- ^ a b "Biopesticides Fact Sheet for Octenol" (PDF). EPA fact sheet. 2007-07-05. Retrieved 2022-06-28.
- ^ "1-octen-3-ol". thegoodscentscompany.com. Retrieved 2015-05-31.
- ^ US FDAs Center for Food Safety and Applied Nutrition. "US FDA/CFSAN – EAFUS List". Archived from the original on 2008-02-21. Retrieved 2008-03-16.
- ^ Inamdar AA, Hossain MM, Bernstein AI, Miller GW, Richardson JR, Bennett JW (November 2013). "Fungal-derived semiochemical 1-octen-3-ol disrupts dopamine packaging and causes neurodegeneration". Proceedings of the National Academy of Sciences of the United States of America. 110 (48): 19561–6. Bibcode:2013PNAS..11019561I. doi:10.1073/pnas.1318830110. PMC 3845153. PMID 24218591.
- ^ Glindemann D, Dietrich A, Staerk HJ, Kuschk P (October 2006). "The two odors of iron when touched or pickled: (skin) carbonyl compounds and organophosphines". Angewandte Chemie. 45 (42): 7006–9. doi:10.1002/anie.200602100. PMID 17009284. S2CID 45055136.