Boger pyridine synthesis: Difference between revisions
→References: cleanup ref |
|||
| (10 intermediate revisions by 7 users not shown) | |||
| Line 1: | Line 1: | ||
The '''Boger pyridine synthesis''' is a [[cycloaddition]] approach to the formation of [[pyridine]]s named after its inventor [[Dale L. Boger]], who first reported it in 1981.<ref>{{cite journal|last1=Boger|first1=Dale L.|last2=Panek|first2=James S.|title=Diels-Alder reaction of heterocyclic azadienes. I. Thermal cycloaddition of 1,2,4-triazine with enamines: simple preparation of substituted pyridines|journal=The Journal of Organic Chemistry|date=May 1981|volume=46|issue=10|pages=2179–2182|doi=10.1021/jo00323a044}}</ref> The reaction is a form of [[Inverse electron-demand Diels–Alder reaction|inverse-electron demand Diels-Alder reaction]] in which an [[enamine]] reacts with a 1,2,4-[[triazine]] to form the pyridine nucleus.<ref>{{cite journal|last1=Boger|first1=Dale L.|title=Diels-Alder reactions of heterocyclic aza dienes. Scope and applications|journal=Chemical Reviews|date=October 1986|volume=86|issue=5|pages=781–793|doi=10.1021/cr00075a004}}</ref><ref>{{cite book|last1=Li|first1=Jie Jack|title=Name Reactions A Collection of Detailed Reaction Mechanisms|date=2002|publisher=Springer-Verlag|location=Berlin|isbn=3-540-43024-5|page=40}}</ref> The reaction is especially useful for accessing pyridines that would be difficult or impossible to access via other methods and has been used in the [[total synthesis]] of several complicated natural products.<ref>{{cite journal|last1=Boger|first1=Dale L.|last2=Boyce|first2=Christopher W.|last3=Labroli|first3=Marc A.|last4=Sehon|first4=Clark A.|last5=Jin|first5=Qing|title=Total Syntheses of Ningalin A, Lamellarin O, Lukianol A, and Permethyl Storniamide A Utilizing Heterocyclic Azadiene Diels−Alder Reactions|journal=Journal of the American Chemical Society|date=January 1999|volume=121|issue=1|pages=54–62|doi=10.1021/ja982078+}}</ref> |
|||
{{Multiple issues|{{refimprove|date=October 2015}}{{more footnotes|date=October 2015}}}} |
|||
The '''Boger pyridine synthesis''' is a cycloaddition approach to the formation of [[Pyridine|pyridines]] in which an [[enamine]] reacts in an [[Inverse electron-demand Diels–Alder reaction|inverse-electron demand Diels-Alder reaction]] with a 1,2,4-[[triazine]] to form the pyridine nucleus. The reaction is especially useful for accessing pyridines that would be difficult or impossible to access via other methods. |
|||
==Mechanism== |
==Mechanism== |
||
| Line 9: | Line 7: | ||
==References== |
==References== |
||
{{Reflist}} |
|||
{{cite journal|last1=Boger|first1=D.|journal=J. Org. Chem.|date=1982|volume=47|page=895}} |
|||
[[Category:Pyridine forming reactions]] |
|||
[[Category:Heterocycle forming reactions]] |
|||
[[Category:Organic reactions]] |
|||
[[Category:Name reactions]] |
|||
{{stub}} |
{{organic-chem-stub}} |
||
Latest revision as of 18:45, 28 July 2016
The Boger pyridine synthesis is a cycloaddition approach to the formation of pyridines named after its inventor Dale L. Boger, who first reported it in 1981.[1] The reaction is a form of inverse-electron demand Diels-Alder reaction in which an enamine reacts with a 1,2,4-triazine to form the pyridine nucleus.[2][3] The reaction is especially useful for accessing pyridines that would be difficult or impossible to access via other methods and has been used in the total synthesis of several complicated natural products.[4]
Mechanism
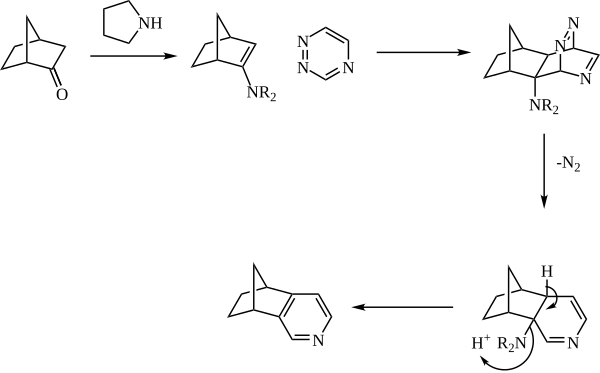
[edit]The enamine is generally generated in situ from catalytic amine (such as pyrrolidine) and a ketone. The enamine then reacts as the dienophile with a 1,2,4-triazine. The initial adduct then expels nitrogen, and the pyridine is rearomatized with loss of the amine.
References
[edit]- ^ Boger, Dale L.; Panek, James S. (May 1981). "Diels-Alder reaction of heterocyclic azadienes. I. Thermal cycloaddition of 1,2,4-triazine with enamines: simple preparation of substituted pyridines". The Journal of Organic Chemistry. 46 (10): 2179–2182. doi:10.1021/jo00323a044.
- ^ Boger, Dale L. (October 1986). "Diels-Alder reactions of heterocyclic aza dienes. Scope and applications". Chemical Reviews. 86 (5): 781–793. doi:10.1021/cr00075a004.
- ^ Li, Jie Jack (2002). Name Reactions A Collection of Detailed Reaction Mechanisms. Berlin: Springer-Verlag. p. 40. ISBN 3-540-43024-5.
- ^ Boger, Dale L.; Boyce, Christopher W.; Labroli, Marc A.; Sehon, Clark A.; Jin, Qing (January 1999). "Total Syntheses of Ningalin A, Lamellarin O, Lukianol A, and Permethyl Storniamide A Utilizing Heterocyclic Azadiene Diels−Alder Reactions". Journal of the American Chemical Society. 121 (1): 54–62. doi:10.1021/ja982078+.
