Influenza A virus: Difference between revisions
Linked term strains |
m Reverted edits by 2400:EC40:102F:E800:989B:459C:FF4F:7E8D (talk) to last version by BD2412 |
||
| (243 intermediate revisions by 74 users not shown) | |||
| Line 1: | Line 1: | ||
{{ |
{{Short description|Species of virus}} |
||
{{cs1 config|name-list-style=vanc|display-authors=6}}{{Use dmy dates|date=May 2024}} |
|||
{{DISPLAYTITLE:''Influenza A virus''}} |
|||
{{Use dmy dates|date=May 2012}} |
|||
{{Virusbox |
{{Virusbox |
||
| image = |
| image = Viruses-12-00504-g001.webp |
||
| |
| image_caption = Structure of influenza A virus |
||
| image2 = Influenza A - late passage.jpg |
|||
| image_caption = [[Transmission electron micrograph]] of influenza A viruses |
|||
| image2_alt = Transmission electron micrograph of influenza A viruses (light objects on a dark background). |
|||
| taxon = Influenza A virus |
|||
| image2_caption = [[Transmission electron micrograph|TEM]] micrograph of influenza A viruses |
|||
| authority = |
|||
| parent = Alphainfluenzavirus |
|||
| synonyms = |
|||
| species = Influenza A virus |
|||
| synonyms_ref = |
|||
| subdivision_ranks = Subtypes |
| subdivision_ranks = Subtypes |
||
| subdivision = |
|||
*[[Influenza A virus subtype H1N1]] |
|||
*[[Influenza A virus subtype H1N2]] |
|||
*[[Influenza A virus subtype H2N2]] |
|||
*[[Influenza A virus subtype H2N3]] |
|||
*[[Influenza A virus subtype H3N1]] |
|||
*[[Influenza A virus subtype H3N2]] |
|||
*[[Influenza A virus subtype H3N8]] |
|||
*[[Influenza A virus subtype H5N1]] |
|||
*[[Influenza A virus subtype H5N2]] |
|||
*[[Influenza A virus subtype H5N3]] |
|||
*[[Influenza A virus subtype H5N6]] |
|||
*[[Influenza A virus subtype H5N8]] |
|||
*[[Influenza A virus subtype H5N9]] |
|||
*[[Influenza A virus subtype H6N1]] |
|||
*[[Influenza A virus subtype H6N2]] |
|||
*[[Influenza A virus subtype H7N1]] |
|||
*[[Influenza A virus subtype H7N2]] |
|||
*[[Influenza A virus subtype H7N3]] |
|||
*[[Influenza A virus subtype H7N4]] |
|||
*[[Influenza A virus subtype H7N7]] |
|||
*[[Influenza A virus subtype H7N9]] |
|||
*[[Influenza A virus subtype H9N2]] |
|||
*[[Influenza A virus subtype H10N7]] |
|||
*[[Influenza A virus subtype H10N8]] |
|||
*[[Influenza A virus subtype H11N2]] |
|||
*[[Influenza A virus subtype H11N9]] |
|||
*[[Influenza A virus subtype H17N10]] |
|||
*[[Influenza A virus subtype H18N11]] |
|||
}} |
}} |
||
''''' |
'''''Influenza A virus''''' (IAV) is the only [[species]] of the [[genus]] ''Alphainfluenzavirus'' of the virus family ''[[Orthomyxoviridae]]''.<ref>{{Cite web |title=Taxonomy |url=https://ictv.global/taxonomy |url-status=live |archive-url=https://web.archive.org/web/20200320103754/https://talk.ictvonline.org/taxonomy |archive-date=20 March 2020 |access-date=19 July 2018 |website=International Committee on Taxonomy of Viruses (ICTV)}}</ref> It is a [[pathogen]] with strains that infect [[Bird|birds]] and some [[mammal]]s, as well as causing [[Flu season|seasonal flu]] in humans.<ref name=":7" /> Mammals in which different strains of IAV circulate with sustained transmission are bats, pigs, horses and dogs; other mammals can occasionally become infected.<ref>{{Cite book | vauthors = Runstadler JA, Puryear W |title=Animal Influenza Virus |date=2020 |chapter=A Brief Introduction to Influenza A Virus in Marine Mammals |chapter-url=https://pubmed.ncbi.nlm.nih.gov/32170708/ |series=Methods in Molecular Biology (Clifton, N.J.) |volume=2123 |pages=429–450 |doi=10.1007/978-1-0716-0346-8_33 |issn=1940-6029 |pmid=32170708|isbn=978-1-0716-0345-1 }}</ref><ref name=":0">{{Cite web |date=2024-05-13 |title=Influenza A Subtypes and the Species Affected {{!}} Seasonal Influenza (Flu) {{!}} CDC |url=https://www.cdc.gov/flu/other/animal-flu.html |access-date=2024-06-17 |website=Centers for Disease Control and Prevention |language=en-us}}</ref> |
||
<!--Virology--> |
|||
IAV is an [[Viral envelope|enveloped]] [[Sense (molecular biology)|negative-sense]] [[RNA virus]], with a segmented genome.<ref name=":0" /> Through a combination of [[mutation]] and genetic [[reassortment]] the virus can evolve to acquire new characteristics, enabling it to evade host immunity and occasionally to jump from one species of host to another.<ref>{{cite journal | vauthors = Shao W, Li X, Goraya MU, Wang S, Chen JL | title = Evolution of Influenza A Virus by Mutation and Re-Assortment | journal = International Journal of Molecular Sciences | volume = 18 | issue = 8 | pages = 1650 | date = August 2017 | pmid = 28783091 | pmc = 5578040 | doi = 10.3390/ijms18081650 | doi-access = free }}</ref><ref name="Eisfeld2">{{cite journal | vauthors = Eisfeld AJ, Neumann G, Kawaoka Y | title = At the centre: influenza A virus ribonucleoproteins | language = En | journal = Nature Reviews. Microbiology | volume = 13 | issue = 1 | pages = 28–41 | date = January 2015 | pmid = 25417656 | pmc = 5619696 | doi = 10.1038/nrmicro3367 }}</ref> |
|||
Influenza A viruses are [[Sense (molecular biology)|negative-sense]], single-stranded, segmented [[RNA virus]]es. |
|||
The several subtypes are labeled according to an H number (for the type of [[Hemagglutinin (influenza)|hemagglutinin]]) and an N number (for the type of [[viral neuraminidase|neuraminidase]]). There are 18 different known H [[antigen]]s (H1 to H18) and 11 different known N antigens (N1 to N11).<ref name="Influenza Subtypes">{{cite web|url=https://www.cdc.gov/flu/avianflu/influenza-a-virus-subtypes.htm|title=Influenza Type A Viruses and Subtypes|publisher=[[Centers for Disease Control and Prevention]]|date=2 April 2013|access-date=13 June 2013}}</ref><ref name="New Influenza Subtypes">{{cite journal | vauthors = Tong S, Zhu X, Li Y, Shi M, Zhang J, Bourgeois M, Yang H, Chen X, Recuenco S, Gomez J, Chen LM, Johnson A, Tao Y, Dreyfus C, Yu W, McBride R, Carney PJ, Gilbert AT, Chang J, Guo Z, Davis CT, Paulson JC, Stevens J, Rupprecht CE, Holmes EC, Wilson IA, Donis RO | title = New world bats harbor diverse influenza A viruses | journal = PLOS Pathogens | volume = 9 | issue = 10 | pages = e1003657 | date = October 2013 | pmid = 24130481 | pmc = 3794996 | doi = 10.1371/journal.ppat.1003657 }}</ref> H17N10 was isolated from [[fruit bat]]s in 2012.<ref>{{cite web|url=http://www.nhs.uk/news/2012/03march/Pages/cdc-finds-h16-bat-influenza.aspx|title=Unique new flu virus found in bats|publisher=NHS Choices|date=1 March 2012|access-date=16 May 2012}}</ref><ref name="pmid22371588">{{cite journal | vauthors = Tong S, Li Y, Rivailler P, Conrardy C, Castillo DA, Chen LM, Recuenco S, Ellison JA, Davis CT, York IA, Turmelle AS, Moran D, Rogers S, Shi M, Tao Y, Weil MR, Tang K, Rowe LA, Sammons S, Xu X, Frace M, Lindblade KA, Cox NJ, Anderson LJ, Rupprecht CE, Donis RO | title = A distinct lineage of influenza A virus from bats | journal = Proceedings of the National Academy of Sciences of the United States of America | volume = 109 | issue = 11 | pages = 4269–74 | date = March 2012 | pmid = 22371588 | pmc = 3306675 | doi = 10.1073/pnas.1116200109 | bibcode = 2012PNAS..109.4269T }}</ref> H18N11 was discovered in a Peruvian bat in 2013.<ref name="New Influenza Subtypes" /> |
|||
Subtypes of IAV are defined by the combination of the antigenic H and N proteins in the [[viral envelope]]; for example, "[[Influenza A virus subtype H1N1|H1N1]]" designates an IAV subtype that has a type-1 hemagglutinin (H) protein and a type-1 neuraminidase (N) protein.<ref name=":112">{{Cite web |last=CDC |date=2024-02-01 |title=Influenza Type A Viruses |url=https://www.cdc.gov/flu/avianflu/influenza-a-virus-subtypes.htm |access-date=2024-05-03 |website=Centers for Disease Control and Prevention |language=en-us}}</ref> Almost all possible combinations of H (1 thru 16) and N (1 thru 11) have been isolated from wild birds.<ref>{{Cite web |title=FluGlobalNet - Avian Influenza |url=https://science.vla.gov.uk/fluglobalnet/about_ai.html |access-date=2024-06-05 |website=science.vla.gov.uk}}</ref> Further variations exist within the subtypes and can lead to very significant differences in the virus's ability to infect and cause disease, as well as to the severity of symptoms.<ref name=":5">{{Cite web |last=CDC |date=2023-03-30 |title=Types of Influenza Viruses |url=https://www.cdc.gov/flu/about/viruses/types.htm |access-date=2024-06-17 |website=Centers for Disease Control and Prevention |language=en-us}}</ref><ref>{{Cite web |last=CDC |date=2024-06-11 |title=Avian Influenza Type A Viruses |url=https://www.cdc.gov/bird-flu/about/index.html |access-date=2024-06-17 |website=Avian Influenza (Bird Flu) |language=en-us}}</ref> |
|||
Each virus subtype has [[Mutation|mutated]] into a variety of strains with differing [[pathogen]]ic profiles; some are pathogenic to one species but not others, some are pathogenic to multiple species. |
|||
<!--Symptoms--> |
|||
Symptoms of human seasonal flu usually include fever, cough, [[sore throat]], [[myalgia|muscle aches]], [[conjunctivitis]] and, in severe cases, breathing problems and [[pneumonia]] that may be fatal.<ref>{{Cite web |date=2017-10-23 |title=Flu |url=https://www.nhs.uk/conditions/flu/ |access-date=2024-06-17 |website=National Health Service |language=en}}</ref><ref name=":7" /> Humans can rarely become infected with strains of [[Avian influenza|avian]] or [[swine influenza]], usually as a result of close contact with infected animals; symptoms range from mild to severe including death.<ref name=":022">{{Cite web |date=2021-11-18 |title=Avian influenza: guidance, data and analysis |url=https://www.gov.uk/government/collections/avian-influenza-guidance-data-and-analysis |access-date=2024-05-09 |website=GOV.UK |language=en}}</ref><ref name=":8">{{Cite web |date=2017-09-20 |title=Swine influenza in humans |url=https://www.ecdc.europa.eu/en/swine-influenza-humans |access-date=2024-06-17 |website=European Centre for Disease Prevention and Control (ECDC) |language=en}}</ref> Bird-adapted strains of the virus can be asymptomatic in some aquatic birds but lethal if they spread to other species, such as chickens.<ref name="joseph">{{cite journal | vauthors = Joseph U, Su YC, Vijaykrishna D, Smith GJ | title = The ecology and adaptive evolution of influenza A interspecies transmission | journal = Influenza and Other Respiratory Viruses | volume = 11 | issue = 1 | pages = 74–84 | date = January 2017 | pmid = 27426214 | pmc = 5155642 | doi = 10.1111/irv.12412 }}</ref> |
|||
A filtered and purified influenza A vaccine for humans has been developed, and many countries have stockpiled it to allow a quick administration to the population in the event of an [[avian influenza]] [[pandemic]]. Avian influenza is sometimes called avian flu, and colloquially, bird flu. In 2011, researchers reported the discovery of an antibody effective against all types of the influenza A virus.<ref name=bbc110729>{{cite news | url = https://www.bbc.co.uk/news/health-14324901 | title = 'Super antibody' fights off flu | last = Gallagher | first = James | name-list-format = vanc | date = 29 July 2011 |work=BBC News | access-date =29 July 2011 }}</ref> |
|||
<!--Treatment and prevention--> |
|||
IAV disease in poultry can be can be prevented by vaccination, however [[biosecurity]] control measures are preferred.<ref>{{Cite web |date=12 June 2024 |title=Avian influenza (bird flu) |url=https://www.ema.europa.eu/en/human-regulatory-overview/public-health-threats/avian-influenza-bird-flu |access-date=2024-06-18 |website=European Medicines Agency}}</ref><ref>{{Cite web |date=5 June 2023 |title=Avian influenza (bird flu) vaccination |url=https://www.gov.uk/government/publications/avian-influenza-bird-flu-vaccination/avian-influenza-bird-flu-vaccination |access-date=2024-06-18 |website=UK Government - Department for Environment Food & Rural Affairs |language=en}}</ref> In humans, seasonal influenza can be treated in its early stages with [[Antiviral drug|antiviral]] medicines.<ref>{{Cite web |last=CDC |date=2024-03-20 |title=What You Should Know about Flu Antiviral Drugs |url=https://www.cdc.gov/flu/treatment/whatyoushould.htm |access-date=2024-06-18 |website=Centers for Disease Control and Prevention |language=en-us}}</ref> A global network, the [[Global Influenza Surveillance and Response System]] (GISRS) monitors the spread of [[influenza]] with the aim to inform development of both seasonal and pandemic vaccines.<ref name="Fange">{{cite book | vauthors = Lee K, Fang J |url=https://books.google.com/books?id=9zCEmpopjG0C&dq=%22WHO%22+%22GISRS+is+a%22&pg=PA163 |title=Historical Dictionary of the World Health Organization |publisher=Rowman & Littlefield |year=2013 |isbn=9780810878587}}</ref> Several millions of specimens are tested by the GISRS network annually through a network of laboratories in 127 countries. As well as human viruses, GISRS monitors avian, swine, and other potentially [[Zoonosis|zoonotic]] influenza viruses. IAV vaccines need to be [[Historical annual reformulations of the influenza vaccine|reformulated regularly]] in order to keep up with changes in the virus.<ref name=":B03">{{Cite web |date=19 September 2022 |title=70 years of GISRS – the Global Influenza Surveillance & Response System |url=https://www.who.int/news-room/feature-stories/detail/seventy-years-of-gisrs---the-global-influenza-surveillance---response-system |access-date=2024-06-13 |website=World Health Organization |language=en}}</ref> |
|||
== Variants and subtypes == |
|||
{{Flu}} |
|||
== Virology == |
|||
{{H5N1}} |
|||
=== Classification === |
|||
Influenza type A viruses are [[RNA virus]]es categorized into subtypes based on the type of two [[proteins]] on the surface of the viral envelope: |
|||
There are two methods of classification, one based on surface proteins (originally [[serotype]]s),<ref name="pmid5309456">{{cite journal | vauthors = Masurel N | title = Serological characteristics of a "new" serotype of influenza A virus: the Hong Kong strain | journal = Bulletin of the World Health Organization | volume = 41 | issue = 3 | pages = 461–468 | date = 1969 | pmid = 5309456 | pmc = 2427714 }}</ref> and the other based on its behavior, mainly the [[Host (biology)|host animal]]. |
|||
=== Subtypes === |
|||
:H = [[hemagglutinin]], a protein that causes [[red blood cells]] to [[Agglutination (biology)|agglutinate]]. |
|||
[[File:InfluenzaNomenclatureDiagram.svg|right|thumb|Diagram of influenza nomenclature]]There are two [[Antigen|antigenic]] [[proteins]] on the surface of the viral envelope, [[hemagglutinin_(influenza)|hemagglutinin]] and [[neuraminidase]].<ref>{{cite journal | vauthors = Johnson J, Higgins A, Navarro A, Huang Y, Esper FL, Barton N, Esch D, Shaw C, Olivo PD, Miao LY | title = Subtyping influenza A virus with monoclonal antibodies and an indirect immunofluorescence assay | journal = Journal of Clinical Microbiology | volume = 50 | issue = 2 | pages = 396–400 | date = February 2012 | pmid = 22075584 | pmc = 3264186 | doi = 10.1128/JCM.01237-11 }}</ref> Different influenza virus genomes encode different hemagglutinin and neuraminidase proteins. Based on their [[serotype]], there are 18 known types of hemagglutinin and 11 types of neuraminidase.<ref name="Influenza Subtypes">{{cite web |date=2 April 2013 |title=Influenza Type A Viruses and Subtypes |url=https://www.cdc.gov/flu/avianflu/influenza-a-virus-subtypes.htm |access-date=13 June 2013 |publisher=[[Centers for Disease Control and Prevention]] |archive-date=1 June 2021 |archive-url=https://web.archive.org/web/20210601122204/https://www.cdc.gov/flu/avianflu/influenza-a-virus-subtypes.htm |url-status=live }}</ref><ref name="New Influenza Subtypes">{{cite journal | vauthors = Tong S, Zhu X, Li Y, Shi M, Zhang J, Bourgeois M, Yang H, Chen X, Recuenco S, Gomez J, Chen LM, Johnson A, Tao Y, Dreyfus C, Yu W, McBride R, Carney PJ, Gilbert AT, Chang J, Guo Z, Davis CT, Paulson JC, Stevens J, Rupprecht CE, Holmes EC, Wilson IA, Donis RO | title = New world bats harbor diverse influenza A viruses | journal = PLOS Pathogens | volume = 9 | issue = 10 | pages = e1003657 | date = October 2013 | pmid = 24130481 | pmc = 3794996 | doi = 10.1371/journal.ppat.1003657 | title-link = doi | doi-access = free }}</ref> Subtypes of IAV are classified by their combination of H and N proteins. For example, "[[influenza A virus subtype H5N1|H5N1]]" designates an influenza A subtype that has a type-5 hemagglutinin (H) protein and a type-1 neuraminidase (N) protein.<ref name="Influenza Subtypes" /> Further variations exist within the subtypes and can lead to very significant differences in the virus's behavior.<ref>{{Cite web |date=2024-02-27 |title=Influenza Virus Genome Sequencing and Genetic Characterization {{!}} CDC |url=https://www.cdc.gov/flu/about/professionals/genetic-characterization.htm |access-date=2024-06-19 |website=Centers for Disease Prevention and Control |language=en-us}}</ref> |
|||
:N = [[neuraminidase]], an enzyme that cleaves the [[glycosidic bond]]s of the [[monosaccharide]] [[sialic acid]] (previously called [[neuraminic acid]]). |
|||
By definition, the subtyping scheme only takes into account the two outer proteins, not the at least 8 proteins internal to the virus.<ref name="Eisfeld">{{cite journal | vauthors = Eisfeld AJ, Neumann G, Kawaoka Y | title = At the centre: influenza A virus ribonucleoproteins | journal = Nature Reviews. Microbiology | volume = 13 | issue = 1 | pages = 28–41 | date = January 2015 | pmid = 25417656 | pmc = 5619696 | doi = 10.1038/nrmicro3367 }}</ref> Almost all possible combinations of H (1 thru 16) and N (1 thru 11) have been isolated from wild birds.<ref name=":4">{{Cite web |title=FluGlobalNet - Avian Influenza |url=https://science.vla.gov.uk/fluglobalnet/about_ai.html |access-date=2024-06-05 |website=science.vla.gov.uk}}</ref> H17 and H18 have only been discovered in bats.<ref>{{Cite web |date=17 June 2024 |title=Influenza A Subtypes and the Species Affected {{!}} Seasonal Influenza (Flu) {{!}} CDC |url=https://www.cdc.gov/flu/other/animal-flu.html |access-date=2024-06-18 |website=Centers for Disease Control and Prevention |language=en-us}}</ref> |
|||
The hemagglutinin is central to the virus's recognizing and binding to target cells, and also to its then infecting the cell with its [[RNA]]. The neuraminidase, on the other hand, is critical for the subsequent release of the daughter virus particles created within the infected cell so they can spread to other cells. |
|||
=== Influenza virus nomenclature === |
|||
Different influenza viruses encode for different hemagglutinin and neuraminidase proteins. For example, the [[influenza A virus subtype H5N1|H5N1 virus]] designates an influenza A subtype that has a type 5 hemagglutinin (H) protein and a type 1 neuraminidase (N) protein. There are 18 known types of hemagglutinin and 11 known types of neuraminidase, so, in theory, 198 different combinations of these proteins are possible.<ref name="Influenza Subtypes" /><ref name="New Influenza Subtypes" /> |
|||
Due to the high variability of the virus, subtyping is not sufficient to uniquely identify a strain of influenza A virus. To unambiguously describe a specific [[Isolation (microbiology)|isolate]] of virus, researchers use the ''Influenza virus nomenclature,<ref>{{cite journal | vauthors = | title = A revision of the system of nomenclature for influenza viruses: a WHO memorandum | journal = Bulletin of the World Health Organization | volume = 58 | issue = 4 | pages = 585–591 | date = 1980 | pmid = 6969132 | pmc = 2395936 | quote = This Memorandum was drafted by the signatories listed on page 590 on the occasion of a meeting held in Geneva in February 1980. }}</ref>'' which describes, among other things, the subtype, year, and place of collection. Some examples include:<ref name=PAHO>{{cite web | title=Technical note: Influenza virus nomenclature | website=Pan American Health Organization | date=11 January 2023 | url=https://www.paho.org/en/documents/technical-note-influenza-virus-nomenclature | access-date=27 May 2024 | archive-date=10 August 2023 | archive-url=https://web.archive.org/web/20230810004651/http://www.paho.org/en/documents/technical-note-influenza-virus-nomenclature | url-status=live }}</ref> |
|||
* {{tt|A/Rio de Janeiro/62434/2021 (H3N2)}}.<ref name=PAHO/> |
|||
Some variants are identified and named according to the isolate they resemble, thus are presumed to share lineage (example [[Fujian flu]] virus-like); according to their typical host (example [[human flu]] virus); according to their subtype (example H3N2); and according to their deadliness (example LP, low pathogenic). So a flu from a virus similar to the isolate A/Fujian/411/2002(H3N2) is called [[Fujian]] flu, human flu, and H3N2 flu. |
|||
** The starting {{tt|A}} indicates that the virus is an influenza A virus. |
|||
** {{tt|Rio de Janeiro}} indicates the place of collection. {{tt|62434}} is a laboratory sequence number. {{tt|2021}} (or just {{tt|21}}) indicates that the sample was collected in 2021. No species is mentioned so by default, the sample was collected from a human. |
|||
** {{tt|(H3N2)}} indicates the subtype of the virus. |
|||
* {{tt|A/swine/South Dakota/152B/2009 (H1N2)}}.<ref name=PAHO/> |
|||
** This example shows an additional field before the place: {{tt|swine}}. It indicates that the sample was collected from a pig. |
|||
* {{tt|A/California/04/2009 A(H1N1)pdm09}}.<ref name=PAHO/> |
|||
** This example carries an unusual designation in the last part: instead of a usual {{tt|(H1N1)}}, it uses {{tt|A(H1N1)pdm09}}. This was in order to distinguish the [[Pandemic H1N1/09 virus]] lineage from older H1N1 viruses.<ref name=PAHO/> |
|||
=== Structure and genetics === |
|||
Variants are sometimes named according to the species (host) in which the strain is endemic or to which it is adapted. The main variants named using this convention are: |
|||
{{See also|H5N1 genetic structure}}[[File:Viruses-10-00497-g001.png|thumb|Influenza A virus structure]] |
|||
* [[Avian influenza|Bird flu]] |
|||
* [[Human flu]] |
|||
* [[Swine influenza]] |
|||
* [[Equine influenza]] |
|||
* [[Canine influenza]] |
|||
==== Structure ==== |
|||
Variants have also sometimes been named according to their deadliness in poultry, especially chickens: |
|||
The influenza A virus has a [[Sense (molecular biology)|negative-sense]], single-stranded, segmented [[RNA virus|RNA genome]], enclosed in a lipid envelope. The virus particle (also called the '''virion''') is 80–120 nanometers in diameter such that the smallest virions adopt an elliptical shape; larger virions have a filamentous shape.<ref>{{cite journal | vauthors = Dadonaite B, Vijayakrishnan S, Fodor E, Bhella D, Hutchinson EC | title = Filamentous influenza viruses | journal = The Journal of General Virology | volume = 97 | issue = 8 | pages = 1755–1764 | date = August 2016 | pmid = 27365089 | pmc = 5935222 | doi = 10.1099/jgv.0.000535 }}</ref> |
|||
* Low pathogenic avian influenza (LPAI) |
|||
* Highly pathogenic avian influenza (HPAI), also called deadly flu or death flu |
|||
Core - The central core of the virion contains the viral RNA genome, which is made of eight separate segments.<ref name="Bouvier, N.M 2008">{{cite journal | vauthors = Bouvier NM, Palese P | title = The biology of influenza viruses | journal = Vaccine | volume = 26 | issue = Suppl 4 | pages = D49–D53 | date = September 2008 | pmid = 19230160 | pmc = 3074182 | doi = 10.1016/j.vaccine.2008.07.039 }}</ref> The nucleoprotein (NP) coats the viral RNA to form a ribonucleoprotein that assumes a helical (spiral) configuration. Three large proteins (PB<sub>1</sub>, PB<sub>2</sub>, and PA), which are responsible for RNA transcription and replication, are bound to each segment of viral RNP.<ref name="Bouvier, N.M 2008" /><ref name=":9">{{Cite web | vauthors = Shaffer C |date=2018-03-07 |title=Influenza A Structure |url=https://www.news-medical.net/life-sciences/Influenza-A-Structure.aspx |access-date=2024-06-18 |website=News-Medical |language=en}}</ref><ref name=":1">{{Cite web |date=13 May 2010 |title=Virology of human influenza |url=https://www.who.int/europe/news-room/fact-sheets/item/virology-of-human-influenza |access-date=2024-06-19 |website=World Health Organization |language=en}}</ref> |
|||
Most known strains are extinct strains. For example, the annual flu subtype H3N2 no longer contains the strain that caused the [[Hong Kong flu]]. |
|||
Capsid - The matrix protein M1 forms a layer between the nucleoprotein and the envelope, called the [[capsid]].<ref name="Bouvier, N.M 2008" /><ref name=":9" /><ref name=":1" /> |
|||
== Annual flu == |
|||
{{main|Flu season}} |
|||
Envelope - The [[viral envelope]] consists of a lipid bilayer derived from the host cell. Two viral proteins; hemagglutinin (HA) and neuraminidase (NA), are inserted into the envelope and are exposed as spikes on the surface of the virion. Both proteins are [[Antigen|antigenic]]; a host's immune system can react to them and produce antibodies in response. The M2 protein forms an ion channel in the envelope and is responsible for uncoating the virion once it has bound to a host cell.<ref name="Bouvier, N.M 2008" /><ref name=":9" /><ref name=":1" /> |
|||
The annual flu (also called "seasonal flu" or "human flu") in the US. "results in approximately 36,000 deaths and more than 200,000 hospitalizations each year. In addition to this human toll, influenza is annually responsible for a total cost of over $10 billion in the U.S."<ref>[http://www.whitehouse.gov/homeland/pandemic-influenza.html whitehouse.gov] {{webarchive|url=https://web.archive.org/web/20090109011356/http://www.whitehouse.gov/homeland/pandemic-influenza.html |date=9 January 2009 }} National Strategy for Pandemic Influenza – Introduction – "Although remarkable advances have been made in science and medicine during the past century, we are constantly reminded that we live in a universe of microbes – viruses, bacteria, protozoa and fungi that are forever changing and adapting themselves to the human host and the defenses that humans create. Influenza viruses are notable for their resilience and adaptability. While science has been able to develop highly effective vaccines and treatments for many infectious diseases that threaten public health, acquiring these tools is an ongoing challenge with the influenza virus. Changes in the genetic makeup of the virus require us to develop new vaccines on an annual basis and forecast which strains are likely to predominate. As a result, and despite annual vaccinations, the US faces a burden of influenza that results in approximately 36,000 deaths and more than 200,000 hospitalizations each year. In addition to this human toll, influenza is annually responsible for a total cost of over $10 billion in the US. A pandemic, or worldwide outbreak of a new influenza virus, could dwarf this impact by overwhelming our health and medical capabilities, potentially resulting in hundreds of thousands of deaths, millions of hospitalizations, and hundreds of billions of dollars in direct and indirect costs. This Strategy will guide our preparedness and response activities to mitigate that impact."</ref> Globally the toll of influenza virus is estimated at 291,000–645,000 deaths annually, exceeding previous estimates.<ref>{{cite journal | vauthors = Iuliano AD, Roguski KM, Chang HH, Muscatello DJ, Palekar R, Tempia S, Cohen C, Gran JM, Schanzer D, Cowling BJ, Wu P, Kyncl J, Ang LW, Park M, Redlberger-Fritz M, Yu H, Espenhain L, Krishnan A, Emukule G, van Asten L, Pereira da Silva S, Aungkulanon S, Buchholz U, Widdowson MA, Bresee JS | title = Estimates of global seasonal influenza-associated respiratory mortality: a modelling study | journal = Lancet | volume = 391 | issue = 10127 | pages = 1285–1300 | date = March 2018 | pmid = 29248255 | pmc = 5935243 | doi = 10.1016/s0140-6736(17)33293-2 }}</ref> |
|||
==== Genome ==== |
|||
The annually updated, trivalent [[influenza vaccine]] consists of [[hemagglutinin]] (HA) surface glycoprotein components from influenza [[Influenza A virus subtype H3N2|H3N2]], [[Influenza A virus subtype H1N1|H1N1]], and [[Influenzavirus B|B influenza]] viruses.<ref>{{cite journal | vauthors = Daum LT, Shaw MW, Klimov AI, Canas LC, Macias EA, Niemeyer D, Chambers JP, Renthal R, Shrestha SK, Acharya RP, Huzdar SP, Rimal N, Myint KS, Gould P | title = Influenza A (H3N2) outbreak, Nepal | journal = Emerging Infectious Diseases | volume = 11 | issue = 8 | pages = 1186–91 | date = August 2005 | pmid = 16102305 | pmc = 3320503 | doi = 10.3201/eid1108.050302 }}<br />"The 2003–2004 influenza season was severe in terms of its impact on illness because of widespread circulation of antigenically distinct influenza A (H3N2) Fujian-like viruses. These viruses first appeared late during the 2002–2003 influenza season and continued to persist as the dominant circulating strain throughout the subsequent 2003–2004 influenza season, replacing the A/Panama/2007/99-like H3N2 viruses (1). Of the 172 H3N2 viruses genetically characterized by the Department of Defense in 2003–2004, only one isolate (from Thailand) belonged to the A/Panama-like lineage. In February 2003, the World Health Organization (WHO) changed the H3N2 component for the 2004–2005 influenza vaccine to afford protection against the widespread emergence of Fujian-like viruses (2). The annually updated trivalent vaccine consists of hemagglutinin (HA) surface glycoprotein components from influenza H3N2, H1N1, and B viruses."</ref> |
|||
The table below presents a concise summary of the influenza genome and the principal functions of the proteins which are encoded. Segments are conventionally numbered from 1 to 8 in descending order of length.<ref name="krammer">{{cite journal | vauthors = Krammer F, Smith GJ, Fouchier RA, Peiris M, Kedzierska K, Doherty PC, Palese P, Shaw ML, Treanor J, Webster RG, García-Sastre A | title = Influenza | journal = Nature Reviews. Disease Primers | volume = 4 | issue = 1 | pages = 3 | date = June 2018 | pmid = 29955068 | pmc = 7097467 | doi = 10.1038/s41572-018-0002-y }}</ref><ref name="Jakob et al">{{cite journal | vauthors = Jakob C, Paul-Stansilaus R, Schwemmle M, Marquet R, Bolte H | title = The influenza A virus genome packaging network - complex, flexible and yet unsolved | journal = Nucleic Acids Research | volume = 50 | issue = 16 | pages = 9023–9038 | date = September 2022 | pmid = 35993811 | doi = 10.1093/nar/gkac688 | pmc = 9458418 }}</ref><ref name="Dou et al">{{cite journal | vauthors = Dou D, Revol R, Östbye H, Wang H, Daniels R | title = Influenza A Virus Cell Entry, Replication, Virion Assembly and Movement | journal = Frontiers in Immunology | volume = 9 | pages = 1581 | date = 2018-07-20 | pmid = 30079062 | pmc = 6062596 | doi = 10.3389/fimmu.2018.01581 | doi-access = free }}</ref><ref name="Rashid et al">{{cite journal | vauthors = Rashid F, Xie Z, Li M, Xie Z, Luo S, Xie L | title = Roles and functions of IAV proteins in host immune evasion | language = English | journal = Frontiers in Immunology | volume = 14 | pages = 1323560 | date = 2023-12-13 | pmid = 38152399 | pmc = 10751371 | doi = 10.3389/fimmu.2023.1323560 | doi-access = free }}</ref> |
|||
{| class="wikitable" |
|||
|RNA Segment |
|||
|Length |
|||
|Protein |
|||
|Function |
|||
|- |
|||
|1- PB2 |
|||
|2341 |
|||
|PB2 (Polymerase Basic 2) |
|||
|A component of the viral RNA [[polymerase]]. |
|||
PB2 also inhibits [[JAK-STAT signaling pathway|JAK1/STAT signaling]] to inhibit host innate immune response |
|||
|- |
|||
| rowspan="2" |2- PB1 |
|||
| rowspan="2" |2341 |
|||
|PB1 (Polymerase Basic 1) |
|||
|A component of the viral RNA polymerase. |
|||
It also degrades the host cell’s [[Mitochondrial antiviral-signaling protein|mitochondrial antiviral signaling protein]] |
|||
|- |
|||
|PB1-F2 (Polymerase Basic 1-Frame 2) |
|||
|An accessory protein of most IAVs. Not needed for virus replication and growth, it interferes with the host immune response. |
|||
|- |
|||
|3- PA |
|||
|2233 |
|||
|PA (Polymerase Acid) |
|||
|A component of the viral RNA polymerase |
|||
|- |
|||
| |
|||
| |
|||
|PA-X |
|||
|Arises from a [[ribosomal frameshift]] in the PA segment. Inhibits innate host immune responses, such as [[cytokine]] and [[interferon]] production. |
|||
|- |
|||
|4- HA |
|||
|1775 |
|||
|[[Hemagglutinin (influenza)|HA (Hemagglutinin)]] |
|||
|Part of the viral envelope, a protein that binds the virion to host cells, enabling the virus’s RNA genetic material to invade it |
|||
|- |
|||
|5- NP |
|||
|1565 |
|||
|[[Nucleoprotein|NP (Nucleoprotein)]] |
|||
|The nucleoprotein associates with the viral RNA to form a ribonucleoprotein (RNP). |
|||
At the early stage of infection, the RNP binds to the host cell’s '''[[Importin α|importin-α]]''' which transports it into the host cell nucleus, where the viral RNA is transcribed and replicated. |
|||
At a later stage of infection, newly manufactured viral RNA segments assemble with the NP protein and polymerase (PB1, PB2 and PA) to form the core of a progeny virion |
|||
Measured resistance to the standard antiviral drugs [[amantadine]] and [[rimantadine]] in H3N2 has increased from 1% in 1994 to 12% in 2003 to 91% in 2005. |
|||
|- |
|||
|6- NA |
|||
|1409 |
|||
|[[Neuraminidase|NA (Neuraminidase)]] |
|||
|Part of the viral envelope. NA enables the newly assembled virions to escape the host cell and go on to propagate the infection. |
|||
NA also facilitates the movement of infective virus particles through mucus, enabling them to reach host epithelial cells. |
|||
|- |
|||
| rowspan="2" |7- M |
|||
| rowspan="2" |1027 |
|||
|[[Viral matrix protein|M1 (Matrix Protein 1)]] |
|||
|Forms the [[capsid]], which coats the viral nucleoproteins and supports the structure of the viral envelope. |
|||
M1 also assists with the function of the NEP protein. |
|||
|- |
|||
|[[M2 proton channel|M2 (Matrix Protein 2)]] |
|||
|Forms a [[Proton pump|proton channel]] in the viral envelope, which is activated once a virion has bound to a host cell. This uncoats the virus, exposing its infective contents to the cytoplasm of the host cell |
|||
|- |
|||
| rowspan="2" |8- NS |
|||
| rowspan="2" |890 |
|||
|[[NS1 influenza protein|NS1 (non-structural protein 1)]] |
|||
|Counteracts the host’s natural immune response and inhibits interferon production. |
|||
|- |
|||
|NEP (Nuclear Export Protein, formerly NS2 non-structural protein 2) |
|||
|Cooperates with the M1 protein to mediate the export of viral RNA copies from nucleus into cytoplasm in the late stage of viral replication |
|||
|} |
|||
Three viral proteins - PB1, PB2, and PA - associate to form the [[RNA-dependent RNA polymerase]] (RdRp) which functions to [[Transcription (biology)|transcribe]] and [[RNA-dependent RNA polymerase|replicate]] the viral RNA. |
|||
'''Viral messenger RNA Transcription -''' The RdRp complex transcribes viral mRNAs by using a mechanism called [[Cap snatching|cap-snatching]]. It consists in the hijacking and cleavage of host [[Five-prime cap|capped]] [[Primary transcript|pre-mRNAs]]. Host cell mRNA is cleaved near the cap to yield a [[Directionality (molecular biology)#5′-end|primer]] for the transcription of positive-sense viral mRNA using the negative-sense viral RNA as a template.<ref>{{cite journal | vauthors = Decroly E, Canard B | title = Biochemical principles and inhibitors to interfere with viral capping pathways | journal = Current Opinion in Virology | volume = 24 | pages = 87–96 | date = June 2017 | pmid = 28527860 | pmc = 7185569 | doi = 10.1016/j.coviro.2017.04.003 }}</ref> The host cell then transports the viral mRNA into the cytoplasm where [[Ribosome|ribosomes]] manufacture the viral proteins.<ref name="krammer" /><ref name="Jakob et al" /><ref name="Dou et al" /><ref name="Rashid et al" /> |
|||
"Contemporary human H3N2 influenza viruses are now [[endemic (epidemiology)|endemic]] in pigs in southern China and can [[reassortment|reassort]] with avian [[Influenza A virus subtype H5N1|H5N1]] viruses in this intermediate host."<ref name=Nap_p126>{{harvnb|Mahmoud|2005|p=[http://www.nap.edu/books/0309095042/html/126.html 126]}}<br />"H5N1 virus is now endemic in poultry in Asia (Table 2-1) and has gained an entrenched ecological niche from which to present a long-term pandemic threat to humans. At present, these viruses are poorly transmitted from poultry to humans, and there is no conclusive evidence of human-to-human transmission. However, continued, extensive exposure of the human population to H5N1 viruses increases the likelihood that the viruses will acquire the necessary characteristics for efficient human-to-human transmission through genetic mutation or reassortment with a prevailing human influenza A virus. Furthermore, contemporary human H3N2 influenza viruses are now endemic in pigs in southern China (Peiris et al., 2001) and can reassort with avian H5N1 viruses in this 'intermediate host.' Therefore, it is imperative that outbreaks of H5N1 disease in poultry in Asia are rapidly and sustainably controlled. The seasonality of the disease in poultry, together with the control measures already implemented, are likely to reduce temporarily the frequency of H5N1 influenza outbreaks and the probability of human infection."</ref> |
|||
'''Replication of the viral RNA -'''The replication of the influenza virus, unlike most other [[Riboviria|RNA viruses]],<ref>{{Cite journal |last=Rampersad |first=Sephra |last2=Tennant |first2=Paula |date=2018 |title=Replication and Expression Strategies of Viruses |url=https://www.ncbi.nlm.nih.gov/pmc/articles/PMC7158166/ |journal=Viruses |pages=55–82 |doi=10.1016/B978-0-12-811257-1.00003-6 |pmc=7158166}}</ref> takes place in the nucleus and involves two steps. The RdRp first of all transcribes the negative-sense viral genome into a positive-sense complimentary RNA (cRNA), then the cRNAs are used as templates to transcribe new negative-sense vRNA copies. These are exported from the nucleus and assemble near the cell membrane to form the core of new virions.<ref name="krammer" /><ref name="Jakob et al" /><ref name="Dou et al" /><ref name="Rashid et al" /> |
|||
===FI6 antibody=== |
|||
[[FI6 (antibody)|FI6]], an [[antibody]] that targets the hemagglutinin protein, was discovered in 2011. FI6 is the only known antibody effective against all 16 subtypes of the influenza A virus.<ref name="BBC">{{cite news|url=https://www.bbc.co.uk/news/health-14324901|title='Super antibody' fights off flu|first=James|last=Gallagher | name-list-format = vanc |date=29 July 2011|via=www.bbc.co.uk|work=BBC News}}</ref><ref name="Independent">{{cite web|url=https://www.independent.co.uk/news/science/scientists-hail-the-prospect-of-a-universal-vaccine-for-flu-2327993.html|title=Scientists hail the prospect of a universal vaccine for flu|date=29 July 2011}}</ref><ref name="Huffington">{{cite web|url=http://www.huffingtonpost.com/2011/07/28/universal-flu-vaccine-antibody_n_912603.html|title=Universal Flu Vaccine On The Horizon: Researchers Find 'Super Antibody'|first=Amanda L.|last=Chan | name-list-format = vanc |date=28 July 2011|via=Huff Post}}</ref> |
|||
[[File:EM of influenza virus.jpg|thumb|A transmission electron micrograph (TEM) of the reconstructed 1918 pandemic influenza virus. The bottom structure represents membrane debris from the cells used to amplify the virus.<ref>{{Cite web|url=https://phil.cdc.gov/details.aspx?pid=8160|title=Details – Public Health Image Library(PHIL)|website=phil.cdc.gov|language=en-us|access-date=2018-04-24}}</ref> Pictured are the 'elliptical' particles representing the smallest particles produced by influenza virus. Purification techniques often deform the particles without proper fixation protocols, leading to 'spherical' appearance.<ref name="Sugita_2011" /> Filamentous or intermediate sized particles simply extend along the long axis on the opposite side of the genome segments.]] |
|||
== Epidemiology == |
|||
==Structure and genetics== |
|||
{{See also|H5N1 genetic structure}} |
|||
Influenza type A viruses are very similar in structure to influenza viruses types B, C, and D.<ref>{{cite journal | vauthors = Nakatsu S, Murakami S, Shindo K, Horimoto T, Sagara H, Noda T, Kawaoka Y | title = Influenza C and D Viruses Package Eight Organized Ribonucleoprotein Complexes | journal = Journal of Virology | volume = 92 | issue = 6 | pages = e02084–17 | date = March 2018 | pmid = 29321324 | pmc = 5827381 | doi = 10.1128/jvi.02084-17 }}</ref> The virus particle (also called the virion) is 80–120 nanometers in diameter such that the smallest virions adopt an elliptical shape.<ref name="pmid22291683">{{cite journal | vauthors = Noda T | title = Native morphology of influenza virions | journal = Frontiers in Microbiology | volume = 2 | issue = | pages = 269 | date = 2011 | pmid = 22291683 | pmc = 3249889 | doi = 10.3389/fmicb.2011.00269 }}</ref><ref name="Sugita_2011">{{cite journal | vauthors = Sugita Y, Noda T, Sagara H, Kawaoka Y | title = Ultracentrifugation deforms unfixed influenza A virions | journal = The Journal of General Virology | volume = 92 | issue = Pt 11 | pages = 2485–93 | date = November 2011 | pmid = 21795472 | pmc = 3352361 | doi = 10.1099/vir.0.036715-0 }}</ref> The length of each particle varies considerably, owing to the fact that influenza is pleomorphic, and can be in excess of many tens of micrometers, producing filamentous virions.<ref name="pmid27365089">{{cite journal | vauthors = Dadonaite B, Vijayakrishnan S, Fodor E, Bhella D, Hutchinson EC | title = Filamentous influenza viruses | journal = The Journal of General Virology | volume = 97 | issue = 8 | pages = 1755–64 | date = August 2016 | pmid = 27365089 | pmc = 5935222 | doi = 10.1099/jgv.0.000535 }}</ref> Confusion about the nature of influenza virus pleomorphy stems from the observation that lab adapted strains typically lose the ability to form filaments<ref name="pmid24089563">{{cite journal | vauthors = Seladi-Schulman J, Steel J, Lowen AC | title = Spherical influenza viruses have a fitness advantage in embryonated eggs, while filament-producing strains are selected in vivo | journal = Journal of Virology | volume = 87 | issue = 24 | pages = 13343–53 | date = December 2013 | pmid = 24089563 | pmc = 3838284 | doi = 10.1128/JVI.02004-13 }}</ref> and that these lab adapted strains were the first to be visualized by electron microscopy.<ref name="pmid21016866">{{cite journal | vauthors = Mosley VM, Wyckoff RW | title = Electron micrography of the virus of influenza | journal = Nature | volume = 157 | issue = 3983| pages = 263 | date = March 1946 | pmid = 21016866 | doi = 10.1038/157263a0| bibcode = 1946Natur.157..263M }}</ref> Despite these varied shapes, the virions of all influenza type A viruses are similar in composition. They are all made up of a viral envelope containing two main types of proteins, wrapped around a central core.<ref name="Bouvier, N.M 2008">{{cite journal | vauthors = Bouvier NM, Palese P | title = The biology of influenza viruses | journal = Vaccine | volume = 26 Suppl 4 | issue = | pages = D49–53 | date = September 2008 | pmid = 19230160 | pmc = 3074182 | doi = 10.1016/j.vaccine.2008.07.039 }}</ref> |
|||
===Evolution and history=== |
|||
The two large proteins found on the outside of viral particles are hemagglutinin (HA) and neuraminidase (NA). HA is a protein that mediates binding of the virion to target cells and entry of the viral genome into the target cell. NA is involved in release from the abundant non-productive attachment sites present in mucus<ref>{{cite journal | vauthors = Cohen M, Zhang XQ, Senaati HP, Chen HW, Varki NM, Schooley RT, Gagneux P | title = Influenza A penetrates host mucus by cleaving sialic acids with neuraminidase | journal = Virology Journal | volume = 10 | pages = 321 | date = November 2013 | pmid = 24261589 | pmc = 3842836 | doi = 10.1186/1743-422x-10-321 }}</ref> as well as the release of progeny virions from infected cells.<ref name="Suzuki, Y 2005">{{cite journal | vauthors = Suzuki Y | title = Sialobiology of influenza: molecular mechanism of host range variation of influenza viruses | journal = Biological & Pharmaceutical Bulletin | volume = 28 | issue = 3 | pages = 399–408 | date = March 2005 | pmid = 15744059 | doi = 10.1248/bpb.28.399 }}</ref> These proteins are usually the targets for antiviral drugs.<ref name="pmid12816348">{{cite journal | vauthors = Wilson JC, von Itzstein M | title = Recent strategies in the search for new anti-influenza therapies | journal = Current Drug Targets | volume = 4 | issue = 5 | pages = 389–408 | date = July 2003 | pmid = 12816348 | doi = 10.2174/1389450033491019 }}</ref> Furthermore, they are also the antigen proteins to which a host's antibodies can bind and trigger an immune response. Influenza type A viruses are categorized into subtypes based on the type of these two proteins on the surface of the viral envelope. There are 16 subtypes of HA and 9 subtypes of NA known, but only H 1, 2 and 3, and N 1 and 2 are commonly found in humans.<ref name="pmid17458769">{{cite journal | vauthors = Lynch JP, Walsh EE | title = Influenza: evolving strategies in treatment and prevention | journal = Seminars in Respiratory and Critical Care Medicine | volume = 28 | issue = 2 | pages = 144–58 | date = April 2007 | pmid = 17458769 | doi = 10.1055/s-2007-976487 }}</ref> |
|||
[[File:Genetic Relationships Among Human and Swine Influenza Viruses, 1918-2009 (7704014350).jpg|thumb|Genetic evolution of human and swine influenza viruses, 1918–2009]]{{Also|Timeline of influenza}} |
|||
The predominant natural reservoir of influenza viruses is thought to be wild waterfowl.<ref>{{Cite book |title=The Threat of Pandemic Influenza: Are We Ready? Workshop Summary. Institute of Medicine (US) Forum on Microbial Threats |publisher=National Academies Press (US) |year=2005 |veditors=Mahmoud SM, Alison M, Knobler SL |location=Washington (DC) |chapter=1, The Story of Influenza. | chapter-url = https://www.ncbi.nlm.nih.gov/books/NBK22148/ }}</ref> The subtypes of influenza A virus are estimated to have diverged 2,000 years ago. Influenza viruses A and B are estimated to have diverged from a single ancestor around 4,000 years ago, while the ancestor of influenza viruses A and B and the ancestor of influenza virus C are estimated to have diverged from a common ancestor around 8,000 years ago.<ref name="origin">{{cite journal | vauthors = Suzuki Y, Nei M | title = Origin and evolution of influenza virus hemagglutinin genes | journal = Molecular Biology and Evolution | volume = 19 | issue = 4 | pages = 501–509 | date = April 2002 | pmid = 11919291 | doi = 10.1093/oxfordjournals.molbev.a004105 | publisher = Ocford Academic | title-link = doi | doi-access = free }}</ref> |
|||
Outbreaks of influenza-like disease can be found throughout recorded history. The first probable record is by [[Hippocrates]] in 142 BCE.<ref name=":2">{{Cite web |title=The History of Influenza |url=https://www.flu.com/Articles/2022/The-History-of-Influenza |access-date=2024-06-20 |website=www.flu.com |language=en}}</ref> The historian Fujikawa listed 46 epidemics of flu-like illness in Japan between 862 and 1868.<ref>{{cite journal | vauthors = Shimizu K | title = [History of influenza epidemics and discovery of influenza virus] | journal = Nihon Rinsho. Japanese Journal of Clinical Medicine | volume = 55 | issue = 10 | pages = 2505–2511 | date = October 1997 | pmid = 9360364 }}</ref> In Europe and the Americas, a number of epidemics were recorded through the [[Middle Ages]] and up to the end of the 19th century.<ref name=":2" />[[File:Viruses-10-00497-g004.png|thumb|Timeline of flu pandemics and epidemics caused by influenza A virus]]In 1918-1919 came the first flu pandemic of the 20th century, known generally as the "''[[Spanish flu]]''", which caused an estimated 20 to 50 million deaths worldwide. It is now known that this was caused by an immunologically novel H1N1 subtype of influenza A.<ref>{{Cite web |date=20 March 2019 |title=CDC Archives : 1918 Pandemic (H1N1 virus) |url=https://archive.cdc.gov/#/details?url=https://www.cdc.gov/flu/pandemic-resources/1918-pandemic-h1n1.html |access-date=2024-06-20 |website=Centers for Disease Control and Prevention}}</ref> The next pandemic took place in 1957, the "''[[1957–1958 influenza pandemic|Asian flu]]''", which was caused by a H2N2 subtype of the virus in which the genome segments coding for HA and NA appeared to have derived from avian influenza strains by reassortment, while the remainder of the genome was descended from the 1918 virus.<ref name=":3">{{cite book | collaboration = Institute of Medicine (US) Forum on Microbial Threats |chapter = The Story of Influenza |date=2005 | title = The Threat of Pandemic Influenza: Are We Ready? Workshop Summary |chapter-url=https://www.ncbi.nlm.nih.gov/books/NBK22148/ |access-date=2024-06-20 |publisher=National Academies Press (US) |language=en | vauthors = Knobler SL, Mack A, Mahmoud A, Lemon SM }}</ref> The 1968 pandemic ("''[[Hong Kong flu]]"'') was caused by a H3N2 subtype in which the NA segment was derived from the 1957 virus, while the HA segment had been reassorted from an avian strain of influenza.<ref name=":3" /> |
|||
The central core of a virion contains the viral genome and other viral proteins that package and protect the genetic material. Unlike the genomes of most organisms (including humans, animals, plants, and bacteria) which are made up of double-stranded DNA, many viral genomes are made up of a different, single-stranded nucleic acid called RNA. Unusually for a virus, though, the influenza type A virus genome is not a single piece of RNA; instead, it consists of segmented pieces of negative-sense RNA, each piece containing either one or two genes which code for a gene product (protein).<ref name="Bouvier, N.M 2008"/> The term negative-sense RNA just implies that the RNA genome cannot be translated into protein directly; it must first be transcribed to positive-sense RNA before it can be translated into protein products. The segmented nature of the genome allows for the exchange of entire genes between different viral strains.<ref name="Bouvier, N.M 2008"/> |
|||
In the 21st century, a strain of H1N1 flu (since titled ''[[Pandemic H1N1/09 virus|H1N1pdm09]]'') which was antigenically very different from previous H1N1 strains, leading to a pandemic in 2009. Because of its close resemblance to some strains circulating in pigs, this became known as "''[[2009 swine flu pandemic|Swine flu]]''"<ref>{{Cite web |date=11 June 2019 |title=2009 H1N1 Pandemic (H1N1pdm09 virus) |url=https://archive.cdc.gov/#/details?url=https://www.cdc.gov/flu/pandemic-resources/2009-h1n1-pandemic.html |access-date=2024-06-21 |website=CDC Archive: Centers for Disease Control and Prevention}}</ref> |
|||
The entire Influenza A virus genome is 13,588 bases long and is contained on eight RNA segments that code for at least 10 but up to 14 proteins, depending on the strain. The relevance or presence of alternate gene products can vary:<ref>{{cite journal | vauthors = Eisfeld AJ, Neumann G, Kawaoka Y | title = At the centre: influenza A virus ribonucleoproteins | language = En | journal = Nature Reviews. Microbiology | volume = 13 | issue = 1 | pages = 28–41 | date = January 2015 | pmid = 25417656 | pmc = 5619696 | doi = 10.1038/nrmicro3367 }}</ref> |
|||
Influenza A virus continues to circulate and evolve in birds and pigs. Almost all possible combinations of H (1 thru 16) and N (1 thru 11) have been isolated from wild birds.<ref name=":4" /> As of June 2024, two particularly virulent IAV strains - [[Influenza A virus subtype H5N1|H5N1]] and [[Influenza A virus subtype H7N9|H7N9]] - are predominant in wild bird populations. These frequently cause outbreaks in domestic poultry, with occasional [[Spillover infection|spillover]] infections in humans who are in close contact with poultry.<ref>{{Cite web |date=26 March 2021 |title=The next pandemic: H5N1 and H7N9 influenza? |url=https://www.gavi.org/vaccineswork/next-pandemic/h5n1-and-h7n9-influenza |access-date=2024-06-21 |website=Gavi, the Vaccine Alliance |language=en}}</ref><ref>{{Cite web |date=3 October 2023 |title=Influenza (Avian and other zoonotic) |url=https://www.who.int/news-room/fact-sheets/detail/influenza-(avian-and-other-zoonotic) |access-date=2024-06-21 |website=World Health Organization |language=en}}</ref> |
|||
* Segment 1 encodes RNA polymerase subunit (PB2). |
|||
* Segment 2 encodes RNA polymerase subunit (PB1) and the PB1-F2 protein, which induces cell death, by using different reading frames from the same RNA segment. |
|||
* Segment 3 encodes RNA polymerase subunit (PA) and the PA-X protein, which has a role in host transcription shutoff.<ref>{{cite journal | vauthors = Khaperskyy DA, Schmaling S, Larkins-Ford J, McCormick C, Gaglia MM | title = Selective Degradation of Host RNA Polymerase II Transcripts by Influenza A Virus PA-X Host Shutoff Protein | journal = PLOS Pathogens | volume = 12 | issue = 2 | pages = e1005427 | date = February 2016 | pmid = 26849127 | pmc = 4744033 | doi = 10.1371/journal.ppat.1005427 }}</ref> |
|||
* Segment 4 encodes for HA (hemagglutinin). About 500 molecules of hemagglutinin are needed to make one virion. HA determines the extent and severity of a viral infection in a host organism. |
|||
* Segment 5 encodes NP, which is a nucleoprotein. |
|||
* Segment 6 encodes NA (neuraminidase). About 100 molecules of neuraminidase are needed to make one virion. |
|||
* Segment 7 encodes two matrix proteins (M1 and M2) by using different reading frames from the same RNA segment. About 3,000 matrix protein molecules are needed to make one virion. |
|||
* Segment 8 encodes two distinct non-structural proteins (NS1 and NEP) by using different reading frames from the same RNA segment. |
|||
=== Pandemic potential === |
|||
The RNA segments of the viral genome have complementary base sequences at the terminal ends, allowing them to bond to each other with hydrogen bonds.<ref name="Suzuki, Y 2005"/> Transcription of the viral (-) sense genome (vRNA) can only proceed after the PB2 protein binds to host capped RNAs, allowing for the PA subunit to cleave several nucleotides after the cap. This host-derived cap and accompanied nucleotides serves as the primer for viral transcription initiation. Transcription proceeds along the vRNA until a stretch of several uracil bases is reached, initiating a 'stuttering' whereby the nascent viral mRNA is poly-adenylated, producing a mature transcript for nuclear export and translation by host machinery.<ref>{{cite journal | vauthors = Te Velthuis AJ, Fodor E | title = Influenza virus RNA polymerase: insights into the mechanisms of viral RNA synthesis | language = En | journal = Nature Reviews. Microbiology | volume = 14 | issue = 8 | pages = 479–93 | date = August 2016 | pmid = 27396566 | doi = 10.1038/nrmicro.2016.87 | pmc = 4966622 }}</ref> |
|||
Influenza viruses have a relatively high mutation rate that is characteristic of [[RNA virus|RNA viruses]].<ref name="SanjuanNebot20102">{{cite journal | vauthors = Sanjuán R, Nebot MR, Chirico N, Mansky LM, Belshaw R | title = Viral mutation rates | journal = Journal of Virology | volume = 84 | issue = 19 | pages = 9733–9748 | date = October 2010 | pmid = 20660197 | pmc = 2937809 | doi = 10.1128/JVI.00694-10 }}</ref> The segmentation of the influenza A virus [[genome]] facilitates [[genetic recombination]] by segment [[reassortment]] in hosts who become infected with two different strains of influenza viruses at the same time.<ref name="Kou22">{{cite journal | vauthors = Kou Z, Lei FM, Yu J, Fan ZJ, Yin ZH, Jia CX, Xiong KJ, Sun YH, Zhang XW, Wu XM, Gao XB, Li TX | title = New genotype of avian influenza H5N1 viruses isolated from tree sparrows in China | journal = Journal of Virology | volume = 79 | issue = 24 | pages = 15460–15466 | date = December 2005 | pmid = 16306617 | pmc = 1316012 | doi = 10.1128/JVI.79.24.15460-15466.2005 }}</ref><ref name="WHOinfluenza22">{{cite journal | vauthors = ((The World Health Organization Global Influenza Program Surveillance Network)) | title = Evolution of H5N1 avian influenza viruses in Asia | journal = Emerging Infectious Diseases | volume = 11 | issue = 10 | pages = 1515–1521 | date = October 2005 | pmid = 16318689 | pmc = 3366754 | doi = 10.3201/eid1110.050644 }} ''Figure 1 shows a diagramatic representation of the genetic relatedness of Asian H5N1 hemagglutinin genes from various isolates of the virus''</ref> With reassortment between strains, an avian strain which does not affect humans may acquire characteristics from a different strain which enable it to infect and pass between humans - a [[Zoonosis|zoonotic]] event.<ref name=":B42">{{Cite web |last=CDC |date=2024-05-15 |title=Transmission of Bird Flu Viruses Between Animals and People |url=https://www.cdc.gov/flu/avianflu/virus-transmission.htm |access-date=2024-06-10 |website=Centers for Disease Control and Prevention |language=en-us}}</ref> It is thought that all influenza A viruses causing outbreaks or [[Pandemic|pandemics]] among humans since the 1900s originated from strains circulating in wild aquatic birds through reassortment with other influenza strains.<ref name=":B02">{{cite journal | vauthors = Taubenberger JK, Morens DM | title = Influenza: the once and future pandemic | journal = Public Health Reports | volume = 125 | issue = Suppl 3 | pages = 16–26 | date = April 2010 | pmid = 20568566 | pmc = 2862331 | doi = 10.1177/00333549101250S305 }}</ref><ref name=":B32">{{cite journal | vauthors = Webster RG, Bean WJ, Gorman OT, Chambers TM, Kawaoka Y | title = Evolution and ecology of influenza A viruses | journal = Microbiological Reviews | volume = 56 | issue = 1 | pages = 152–179 | date = March 1992 | pmid = 1579108 | pmc = 372859 | doi = 10.1128/mr.56.1.152-179.1992 }}</ref> It is possible (though not certain) that pigs may act as an intermediate host for reassortment.<ref>{{Cite web |date=2017-06-15 |title=Factsheet on swine influenza in humans and pigs |url=https://www.ecdc.europa.eu/en/swine-influenza/factsheet |access-date=2024-06-13 |website=European Centre for Disease Control |language=en}}</ref> |
|||
=== Surveillance === |
|||
The RNA synthesis takes place in the cell nucleus, while the synthesis of proteins takes place in the cytoplasm. Once the viral proteins are assembled into virions, the assembled virions leave the nucleus and migrate towards the cell membrane.<ref name="Smith, A.E 2004">{{cite journal | vauthors = Smith AE, Helenius A | title = How viruses enter animal cells | journal = Science | volume = 304 | issue = 5668 | pages = 237–42 | date = April 2004 | pmid = 15073366 | doi = 10.1126/science.1094823 | bibcode = 2004Sci...304..237S }}</ref> The host cell membrane has patches of viral transmembrane proteins (HA, NA, and M2) and an underlying layer of the M1 protein which assist the assembled virions to budding through the membrane, releasing finished enveloped viruses into the extracellular fluid.<ref name="Smith, A.E 2004"/> |
|||
The '''[[Global Influenza Surveillance and Response System]] (GISRS)''' is a global network of laboratories that monitor the spread of [[influenza]] with the aim to provide the [[World Health Organization]] with influenza control information and to inform vaccine development.<ref name="Fange" /> Several millions of specimens are tested by the GISRS network annually through a network of laboratories in 127 countries.<ref name=":B033">{{Cite web |date=19 September 2022 |title=70 years of GISRS – the Global Influenza Surveillance & Response System |url=https://www.who.int/news-room/feature-stories/detail/seventy-years-of-gisrs---the-global-influenza-surveillance---response-system |access-date=2024-06-13 |website=World Health Organization |language=en}}</ref> As well as human viruses, GISRS monitors avian, swine, and other potentially [[Zoonosis|zoonotic]] influenza viruses. |
|||
=== Seasonal flu === |
|||
==Multiplicity reactivation== |
|||
{{Main|Flu season}} |
|||
Influenza virus is able to undergo multiplicity reactivation after inactivation by UV radiation,<ref>{{cite journal | vauthors = Barry RD | title = The multiplication of influenza virus. II. Multiplicity reactivation of ultraviolet irradiated virus | journal = Virology | volume = 14 | issue = 4 | pages = 398–405 | date = August 1961 | pmid = 13687359 | doi = 10.1016/0042-6822(61)90330-0 | hdl = 1885/109240 }}</ref><ref>{{cite journal | vauthors = Henle W, Liu OC | title = Studies on host-virus interactions in the chick embryo-influenza virus system. VI. Evidence for multiplicity reactivation of inactivated virus | journal = The Journal of Experimental Medicine | volume = 94 | issue = 4 | pages = 305–22 | date = October 1951 | pmid = 14888814 | pmc = 2136114 | doi = 10.1084/jem.94.4.305 }}</ref> or by ionizing radiation.<ref>{{cite journal | vauthors = Gilker JC, Pavilanis V, Ghys R | title = Multiplicity reactivation in gamma irradiated influenza viruses | journal = Nature | volume = 214 | issue = 5094 | pages = 1235–7 | date = June 1967 | pmid = 6066111 | doi = 10.1038/2141235a0 | bibcode = 1967Natur.214.1235G }}</ref> If any of the eight RNA strands that make up the genome contains damage that prevents replication or expression of an essential gene, the virus is not viable when it alone infects a cell (a single infection). However, when two or more damaged viruses infect the same cell (multiple infection), viable progeny viruses can be produced provided each of the eight genomic segments is present in at least one undamaged copy. That is, multiplicity reactivation can occur. |
|||
[[File:CDC-influenza-pneumonia-deaths-2015-01-10.gif|thumb|'''Seasonal variation in deaths due to influenza or pneumonia in 122 U.S. cities, as a proportion of all causes.<ref name="weekly">[https://www.cdc.gov/flu/weekly/ CDC U.S. influenza season summary with weekly updates] See section 'Pneumonia and Influenza (P&I) Mortality Surveillance' ''www.cdc.gov'', accessed 30 September 2020</ref>''']] |
|||
'''Flu season''' is an annually recurring time period characterized by the prevalence of an outbreak of [[influenza]], caused either by Influenza A or by [[Influenza B virus|Influenza B]]. The season occurs during the cold half of the year in temperate regions; November through February in the northern hemisphere and May to October in the southern hemisphere. Flu seasons also exist in the [[tropics]] and [[subtropics]], with variability from region to region.<ref>{{cite journal | vauthors = Hirve S, Newman LP, Paget J, Azziz-Baumgartner E, Fitzner J, Bhat N, Vandemaele K, Zhang W | title = Influenza Seasonality in the Tropics and Subtropics - When to Vaccinate? | journal = PLOS ONE | volume = 11 | issue = 4 | pages = e0153003 | date = 2016-04-27 | pmid = 27119988 | pmc = 4847850 | doi = 10.1371/journal.pone.0153003 | doi-access = free | bibcode = 2016PLoSO..1153003H }}</ref> Annually, about 3 to 5 million cases of severe illness and 290,000 to 650,000 deaths from seasonal flu occur worldwide.<ref name=":7" /> |
|||
There are several possible reasons for the winter peak in temperate regions: |
|||
Upon infection, influenza virus induces a host response involving increased production of reactive oxygen species, and this can damage the virus genome.<ref>{{cite journal | vauthors = Peterhans E | title = Oxidants and antioxidants in viral diseases: disease mechanisms and metabolic regulation | journal = The Journal of Nutrition | volume = 127 | issue = 5 Suppl | pages = 962S–965S | date = May 1997 | pmid = 9164274 | doi = 10.1093/jn/127.5.962S}}</ref> If, under natural conditions, virus survival is ordinarily vulnerable to the challenge of oxidative damage, then multiplicity reactivation is likely selectively advantageous as a kind of genomic repair process. It has been suggested that multiplicity reactivation involving segmented RNA genomes may be similar to the earliest evolved form of sexual interaction in the RNA world that likely preceded the DNA world.<ref>{{cite journal | vauthors = Bernstein H, Byerly HC, Hopf FA, Michod RE | title = Origin of sex | journal = Journal of Theoretical Biology | volume = 110 | issue = 3 | pages = 323–51 | date = October 1984 | pmid = 6209512 | doi = 10.1016/S0022-5193(84)80178-2 }}</ref> (Also see [[RNA world hypothesis]].) |
|||
* During the winter, people spend more time indoors with the windows sealed, so they are more likely to breathe the same air as someone who has the flu and thus contract the virus.<ref name=":02">{{Cite web |date=1 December 2014 |title=The Reason for the Season: why flu strikes in winter |url=https://sitn.hms.harvard.edu/flash/2014/the-reason-for-the-season-why-flu-strikes-in-winter/ |access-date=21 June 2024 |website=Science in the News, a Graduate Student Group at the Harvard Graduate School of the Arts and Sciences. |language=en-US}}</ref> |
|||
==Human influenza virus== |
|||
* Days are shorter during the winter, and lack of sunlight leads to low levels of vitamin D and melatonin, both of which require sunlight for their generation. This compromises our immune systems, which in turn decreases ability to fight the virus.<ref name=":02" /> |
|||
* The influenza virus may survive better in colder, drier climates, and therefore be able to infect more people.<ref name=":02" /> |
|||
* Cold air reduces the ability of the nasal membranes to resist infection.<ref>{{Cite web | vauthors = LaMotte S |date=2022-12-06 |title=Scientists finally know why people get more colds and flu in winter |url=https://www.cnn.com/2022/12/06/health/why-winter-colds-flu-wellness/index.html |access-date=2024-06-21 |website=CNN |language=en}}</ref> |
|||
=== Zoonotic infections === |
|||
"Human influenza virus" usually refers to those subtypes that spread widely among humans. H1N1, H1N2, and H3N2 are the only known influenza A virus subtypes currently circulating among humans.<ref>[https://www.cdc.gov/flu/avian/gen-info/facts.htm CDC] ''Key Facts About Avian Influenza (Bird Flu) and Avian Influenza A (H5N1) Virus''</ref> |
|||
A [[zoonosis]] a disease in a human caused by a [[pathogen]] (such as a [[bacterium]], or [[virus]]) that has jumped from a non-human to a [[human]].<ref name="mw">{{cite Merriam-Webster|zoonosis|access-date=29 March 2019}}</ref><ref name=":03">{{Cite web |date=29 July 2020 |title=Zoonoses - Key Facts |url=https://www.who.int/news-room/fact-sheets/detail/zoonoses |access-date=2024-06-24 |website=World Health Organization |language=en}}</ref> Avian and pig influenza viruses can, on rare occasions, transmit to humans and cause zoonotic influenza virus infections; these infections are usually confined to people who have been in close contact with infected animals or material such as infected feces and meat, they do not spread to other humans. Symptoms of these infections in humans vary greatly; some are in asymptomatic or mild while others can cause severe disease, leading to severe pneumonia and death.<ref name=":12">{{Cite web |date=2023-05-23 |title=Zoonotic influenza - Annual Epidemiological Report for 2022 |url=https://www.ecdc.europa.eu/en/publications-data/zoonotic-influenza-annual-epidemiological-report-2022 |access-date=2024-06-24 |website=www.ecdc.europa.eu |language=en}}</ref> A wide range of Influenza A virus subtypes have been found to cause zoonotic disease.<ref name=":12" /><ref name=":22">{{Cite web |date=29 July 2020 |title=Global AIV with Zoonotic Potential |url=https://www.fao.org/animal-health/situation-updates/global-aiv-with-zoonotic-potential/en |access-date=2024-06-24 |website=The Food and Agriculture Organization (FAO) of the United Nations |language=en}}</ref> |
|||
Zoonotic infections can be prevented by good hygiene, by preventing farmed animals from coming into contact with wild animals, and by using appropriate personal protective equipment.<ref name=":03" /> |
|||
Genetic factors in distinguishing between "human flu viruses" and "avian influenza viruses" include: |
|||
:'''PB2''': (RNA polymerase): [[Amino acid]] (or [[residue (chemistry)|residue]]) position 627 in the PB2 protein encoded by the PB2 RNA gene. Until H5N1, all known avian influenza viruses had a [[Glutamic acid|Glu]] at position 627, while all human influenza viruses had a [[lysine]]. |
|||
:'''HA''': (hemagglutinin): Avian influenza HA binds alpha 2–3 [[sialic acid]] receptors, while human influenza HA binds alpha 2–6 sialic acid receptors. Swine influenza viruses have the ability to bind both types of sialic acid receptors. |
|||
As of June 2024, there is concern about two subtypes of avian influenza which are circulating in wild bird populations worldwide, [[Influenza A virus subtype H5N1|H5N1]] and [[Influenza A virus subtype H7N9|H7N9]]. Both of these have potential to devastate poultry stocks, and both have jumped to humans with relatively high [[Case fatality rate|case fatality rates]].<ref name=":22" /> H5N1 in particular has infected a [[List of mammals that can get H5N1|wide range of mammals]] and may be adapting to mammalian hosts.<ref>{{cite journal | vauthors = Plaza PI, Gamarra-Toledo V, Euguí JR, Lambertucci SA | title = Recent Changes in Patterns of Mammal Infection with Highly Pathogenic Avian Influenza A(H5N1) Virus Worldwide | language = en-us | journal = Emerging Infectious Diseases | volume = 30 | issue = 3 | pages = 444–452 | date = March 2024 | pmid = 38407173 | pmc = 10902543 | doi = 10.3201/eid3003.231098 }}</ref> |
|||
"About 52 key genetic changes distinguish avian influenza strains from those that spread easily among people, according to researchers in Taiwan, who analyzed the genes of more than 400 A type flu viruses."<ref>[https://www.bloomberg.com/apps/news?pid=20601086&sid=a6S3ZQwqZkS4&refer=latin_america Bloomberg News] article '' Scientists Move Closer to Understanding Flu Virus Evolution'' published 28 August 2006</ref> "How many mutations would make an avian virus capable of infecting humans efficiently, or how many mutations would render an influenza virus a pandemic strain, is difficult to predict. We have examined sequences from the 1918 strain, which is the only pandemic influenza virus that could be entirely derived from avian strains. Of the 52 species-associated positions, 16 have residues typical for human strains; the others remained as avian signatures. The result supports the hypothesis that the 1918 pandemic virus is more closely related to the avian influenza A virus than are other human influenza viruses."<ref>{{cite journal | vauthors = Chen GW, Chang SC, Mok CK, Lo YL, Kung YN, Huang JH, Shih YH, Wang JY, Chiang C, Chen CJ, Shih SR | title = Genomic signatures of human versus avian influenza A viruses | journal = Emerging Infectious Diseases | volume = 12 | issue = 9 | pages = 1353–60 | date = September 2006 | pmid = 17073083 | pmc = 3294750 | doi = 10.3201/eid1209.060276 }}</ref> |
|||
== Prevention and treatment == |
|||
Human flu symptoms usually include fever, cough, [[sore throat]], [[myalgia|muscle aches]], [[conjunctivitis]] and, in severe cases, severe breathing problems and [[pneumonia]] that may be fatal. The severity of the infection will depend in large part on the state of the infected person's [[immune system]] and if the victim has been exposed to the strain before, and is therefore partially immune. Recent follow up studies on the impact of statins on influenza virus replication show that pre-treatment of cells with atorvastatin suppresses virus growth in culture.<ref>{{cite journal | vauthors = Episcopio D, Aminov S, Benjamin S, Germain G, Datan E, Landazuri J, Lockshin RA, Zakeri Z | title = Atorvastatin restricts the ability of influenza virus to generate lipid droplets and severely suppresses the replication of the virus | journal = The FASEB Journal | volume = 33 | issue = 8 | pages = 9516–9525 | date = April 2019 | pmid = 31125254 | pmc = 6662987 | doi = 10.1096/fj.201900428RR }}</ref> |
|||
=== Vaccine === |
|||
Highly pathogenic H5N1 avian influenza in a human is far worse, killing 50% of humans who catch it. In one case, a boy with H5N1 experienced [[diarrhea]] followed rapidly by a coma without developing respiratory or flu-like symptoms.<ref>{{cite journal | vauthors = de Jong MD, Bach VC, Phan TQ, Vo MH, Tran TT, Nguyen BH, Beld M, Le TP, Truong HK, Nguyen VV, Tran TH, Do QH, Farrar J | title = Fatal avian influenza A (H5N1) in a child presenting with diarrhea followed by coma | journal = The New England Journal of Medicine | volume = 352 | issue = 7 | pages = 686–91 | date = February 2005 | pmid = 15716562 | doi = 10.1056/NEJMoa044307 | url = https://semanticscholar.org/paper/f40c52088d0dac1610a2cf8a90fa82f472706acd }}</ref> |
|||
{{Main|Influenza vaccine}} |
|||
As of June 2024, the influenza viruses which circulate widely in humans are IAV subtypes H1N1 and H2N3, together with Influenza B.<ref>{{Cite web |last=CDC |date=2023-03-30 |title=Types of Influenza Viruses |url=https://www.cdc.gov/flu/about/viruses/types.htm |access-date=2024-06-22 |website=Centers for Disease Control and Prevention |language=en-us}}</ref> Annual vaccination is the primary and most effective way to prevent influenza and influenza-associated complications, especially for high-risk groups.<ref name="chow">{{cite journal | vauthors = Chow EJ, Doyle JD, Uyeki TM | title = Influenza virus-related critical illness: prevention, diagnosis, treatment | journal = Critical Care | volume = 23 | issue = 1 | pages = 214 | date = June 2019 | pmid = 31189475 | pmc = 6563376 | doi = 10.1186/s13054-019-2491-9 | doi-access = free }}</ref> Vaccines against the flu are trivalent or quadrivalent, providing protection against the dominant strains of IAV(H1N1) and IAV(H3N2), and one or two influenza B virus strains; the formulation is continually reviewed in order to match the predominant strains in circulation.<ref name="dharmapalan">{{cite journal | vauthors = Dharmapalan D | title = Influenza | journal = Indian Journal of Pediatrics | volume = 87 | issue = 10 | pages = 828–832 | date = October 2020 | pmid = 32048225 | pmc = 7091034 | doi = 10.1007/s12098-020-03214-1 }}</ref><ref name="sautto">{{cite journal | vauthors = Sautto GA, Kirchenbaum GA, Ross TM | title = Towards a universal influenza vaccine: different approaches for one goal | journal = Virology Journal | volume = 15 | issue = 1 | pages = 17 | date = January 2018 | pmid = 29370862 | pmc = 5785881 | doi = 10.1186/s12985-017-0918-y | doi-access = free }}</ref> |
|||
'''Poultry and other animals''' - it is possible to vaccinate poultry and pigs against specific strains of influenza. Vaccination should be combined with other control measures such as infection monitoring, early detection and biosecurity.<ref>{{Cite web |date=2023-10-10 |title=Vaccination of poultry against highly pathogenic avian influenza – Available vaccines and vaccination strategies |url=https://www.efsa.europa.eu/en/news/vaccination-poultry-against-highly-pathogenic-avian-influenza-available-vaccines-and |access-date=2024-05-09 |website=efsa.europa.eu |publisher= |language=en}}</ref><ref>{{Cite web |date=2024-06-03 |title=Making a Candidate Vaccine Virus (CVV) for a HPAI (Bird Flu) Virus |url=https://www.cdc.gov/bird-flu/php/severe-potential/candidate-vaccine-virus.html |access-date=2024-06-15 |website=Centers for Disease Control |language=en-us}}</ref><ref>{{Cite web |date=2023-10-19 |title=What People Who Raise Pigs Need To Know About Influenza (Flu) {{!}} CDC |url=https://www.cdc.gov/flu/swineflu/people-raise-pigs-flu.htm |access-date=2024-06-22 |website=Centers for Disease Control and Prevention |language=en-us}}</ref> |
|||
The influenza A virus subtypes that have been confirmed in humans, ordered by the number of known human pandemic deaths, are: |
|||
* [[H1N1]] caused "[[Spanish flu]]" in 1918 and the [[2009 swine flu outbreak]] |
|||
* [[H2N2]] caused "[[Asian Flu|Asian flu]]" in the late 1950s |
|||
* [[H3N2]] caused "[[Hong Kong flu]]" in the late 1960s |
|||
* [[H5N1]] considered a global [[influenza pandemic]] threat through [[Global spread of H5N1|its spread]] in the mid-2000s |
|||
* [[H7N9]] is responsible for an ongoing epidemic in China and considered to have the greatest pandemic threat of the Influenza A viruses |
|||
* [[H7N7]] has unusual [[zoonotic]] potential |
|||
* [[H1N2]] is currently endemic in humans{{Citation needed|date=April 2018}} and pigs |
|||
* [[H9N2]], [[H7N2]], [[H7N3]], [[H5N2]], and [[H10N7]]. |
|||
=== Treatment === |
|||
;H1N1 |
|||
{{ |
{{Main|Influenza treatment}} |
||
The main treatment for mild influenza is supportive; rest, fluids, and over-the-counter medicines to alleviate symptoms while the body's own [[immune system]] works to recover from infection. [[Antiviral drug|Antiviral drugs]] are recommended for those with severe symptoms, or for those who are at risk of developing complications such as pneumonia.<ref>{{Cite web |last=CDC |date=2024-03-22 |title=Take everyday precautions to protect others while sick |url=https://t.cdc.gov/2S4E |access-date=2024-06-22 |website=Centers for Disease Control and Prevention |language=en-us}}</ref><ref name=":7">{{Cite web |title=Influenza (Seasonal) |url=https://www.who.int/news-room/fact-sheets/detail/influenza-(seasonal) |access-date=2024-06-22 |website=World Health Organization |language=en}}</ref> |
|||
:H1N1 is currently pandemic in both human and pig populations. A variant of H1N1 was responsible for the Spanish flu pandemic that killed some 50 million to 100 million people worldwide over about a year in 1918 and 1919.<ref>{{harvnb|Mahmoud|2005|p=[http://www.nap.edu/books/0309095042/html/7.html 7]}}</ref> Another variant was named a pandemic threat in the [[2009 flu pandemic]]. Controversy arose in October 2005, after the H1N1 [[genome]] was published in the journal, [[Science (journal)|''Science'']], because of fears that this information could be used for [[bioterrorism]].{{Citation needed|date=December 2010}} |
|||
== Signs and symptoms == |
|||
;H1N2 |
|||
{{Main|influenza}} |
|||
{{main|Influenza A virus subtype H1N2}} |
|||
:H1N2 is currently endemic in both human {{Citation needed|date=April 2018}} and pig populations. The new H1N2 strain appears to have resulted from the reassortment of the genes of the currently circulating influenza H1N1 and H3N2 subtypes. The hemagglutinin protein of the H1N2 virus is similar to that of the currently circulating H1N1 viruses, and the neuraminidase protein is similar to that of the current H3N2 viruses. |
|||
=== Humans === |
|||
;H2N2 |
|||
[[File:Symptoms_of_influenza.svg|thumb|Symptoms of influenza,<ref>{{cite web |date=10 July 2019 |title=Flu Symptoms & Diagnosis |url=https://www.cdc.gov/flu/symptoms/index.html |url-status=live |archive-url=https://web.archive.org/web/20191227211854/https://www.cdc.gov/flu/symptoms/index.html |archive-date=27 December 2019 |access-date=24 January 2020 |website=U.S. [[Centers for Disease Control and Prevention]] (CDC)}}</ref><ref>{{cite web |date=26 February 2019 |title=Flu Symptoms & Complications |url=https://www.cdc.gov/flu/symptoms/symptoms.htm |url-status=live |archive-url=https://web.archive.org/web/20200801071343/https://www.cdc.gov/flu/symptoms/symptoms.htm |archive-date=1 August 2020 |access-date=6 July 2019 |publisher=U.S. [[Centers for Disease Control and Prevention]] (CDC)}}</ref> with fever and cough the most common symptoms.<ref name="pmid15728170">{{cite journal | vauthors = Call SA, Vollenweider MA, Hornung CA, Simel DL, McKinney WP | title = Does this patient have influenza? | journal = JAMA | volume = 293 | issue = 8 | pages = 987–997 | date = February 2005 | pmid = 15728170 | doi = 10.1001/jama.293.8.987 }}</ref>]] |
|||
{{main|Influenza A virus subtype H2N2}} |
|||
The symptoms of seasonal flu are similar to those of a [[Common cold|cold]], although usually more severe and less likely to include a [[runny nose]].<ref>{{Cite web |last=CDC |date=2022-09-29 |title=Cold Versus Flu |url=https://www.cdc.gov/flu/symptoms/coldflu.htm |access-date=2024-06-25 |website=Centers for Disease Control and Prevention |language=en-us}}</ref> The onset of symptoms is sudden, and initial symptoms are predominately non-specific: a sudden fever; muscle aches; cough; fatigue; sore throat; headache; difficulty sleeping; loss of appetite; diarrhoea or abdominal pain; nausea and vomiting.<ref>{{Cite web |date=9 August 2023 |title=Flu |url=https://www.nhs.uk/conditions/flu/ |access-date=2024-06-25 |website=National Health Service UK |language=en-GB}}</ref> |
|||
:The Asian flu, a pandemic outbreak of H2N2 avian influenza, originated in China in 1957, spread worldwide that same year during which an influenza vaccine was developed, lasted until 1958 and caused between one and four million deaths. |
|||
Humans can rarely become infected with strains of [[Avian influenza|avian]] or [[swine influenza]], usually as a result of close contact with infected animals or contaminated material; symptoms generally resemble seasonal flu but occasionally can be severe including death.<ref name=":022" /><ref name=":8" /> |
|||
;H3N2 |
|||
{{main|Influenza A virus subtype H3N2}} |
|||
:H3N2 is currently endemic in both human and pig populations. It evolved from H2N2 by [[antigenic shift]] and caused the Hong Kong flu pandemic of 1968 and 1969 that killed up to 750,000.<ref>Detailed chart of its evolution [http://www.eletrica.ufsj.edu.br/~nepomuceno/references/epidemiology/ear_eal02.pdf here] {{webarchive|url=https://web.archive.org/web/20090509025529/http://www.eletrica.ufsj.edu.br/~nepomuceno/references/epidemiology/ear_eal02.pdf |date=9 May 2009 }} at PDF called ''Ecology and Evolution of the Flu''</ref> "An early-onset, severe form of influenza A H3N2 made headlines when it claimed the lives of several children in the United States in late 2003."<ref>{{harvnb|Mahmoud|2005|p=[http://www.nap.edu/books/0309095042/html/115.html 115]}}<br />"There is particular pressure to recognize and heed the lessons of past influenza pandemics in the shadow of the worrisome 2003–2004 flu season. An early-onset, severe form of influenza A H3N2 made headlines when it claimed the lives of several children in the United States in late 2003. As a result, stronger than usual demand for annual flu inactivated vaccine outstripped the vaccine supply, of which 10 to 20 percent typically goes unused. Because statistics on pediatric flu deaths had not been collected previously, it is unknown if the 2003–2004 season witnessed a significant change in mortality patterns."</ref> |
|||
=== Other animals === |
|||
:The dominant strain of annual flu in January 2006 was H3N2. Measured resistance to the standard antiviral drugs amantadine and rimantadine in H3N2 increased from 1% in 1994 to 12% in 2003 to 91% in 2005.<ref>[http://www.reason.com/rb/rb101905.shtml Reason] {{webarchive|url=https://web.archive.org/web/20061026092508/http://www.reason.com/rb/rb101905.shtml |date=26 October 2006 }} [https://www.nytimes.com/2006/01/15/health/15drugs.html New York Times] ''This Season's Flu Virus Is Resistant to 2 Standard Drugs'' By Altman Published: 15 January 2006</ref> |
|||
==== Birds ==== |
|||
:"[C]ontemporary human H3N2 influenza viruses are now endemic in pigs in southern China and can reassort with avian H5N1 viruses in this intermediate host."<ref name=Nap_p126/> |
|||
{{Main|Avian influenza}} |
|||
Some species of wild [[Water bird|aquatic birds]] act as natural [[asymptomatic carrier]]s of a large variety of influenza A viruses, which they can spread over large distances in their annual migration.<ref>{{Cite web |date=2022-12-13 |title=Bird flu (avian influenza): how to spot and report it in poultry or other captive birds |url=https://www.gov.uk/guidance/avian-influenza-bird-flu |access-date=2024-05-06 |website=Department for Environment, Food & Rural Affairs and Animal and Plant Health Agency |language=en}}</ref> Symptoms of avian influenza vary according to both the strain of virus underlying the infection, and on the species of bird affected. Symptoms of influenza in birds may include swollen head, watery eyes, unresponsiveness, lack of coordination, respiratory distress such as sneezing or gurgling.<ref>{{Cite web |title=Avian flu |url=https://rspb.org.uk/birds-and-wildlife/avian-influenza-updates |access-date=2024-06-25 |website=The Royal Society for the Protection of Birds (RSPB)}}</ref> |
|||
;H5N1 |
|||
{{main|Influenza A virus subtype H5N1}} |
|||
:H5N1 is the world's major influenza pandemic threat. |
|||
===== Highly pathogenic avian influenza ===== |
|||
:"When he compared the [[1918 flu pandemic|1918 virus]] with today's human flu viruses, Dr. Taubenberger noticed that it had alterations in just 25 to 30 of the virus's 4,400 amino acids. Those few changes turned a bird virus into a killer that could spread from person to person."<ref>[https://www.nytimes.com/2005/11/08/science/08flu.html?pagewanted=2 New York Times] Published: 8 November 2005 – ''Hazard in Hunt for New Flu: Looking for Bugs in All the Wrong Places''</ref> |
|||
Because of the impact of avian influenza on economically important chicken farms, avian virus strains are classified as either highly pathogenic (and therefore potentially requiring vigorous control measures) or low pathogenic. The test for this is based solely on the effect on chickens - a virus strain is '''highly pathogenic avian influenza''' ('''HPAI''') if 75% or more of chickens die after being deliberately infected with it, or if it is genetically similar to such a strain. The alternative classification is '''low pathogenic avian influenza''' (LPAI).<ref name=":AA6">{{cite journal | vauthors = Alexander DJ, Brown IH | title = History of highly pathogenic avian influenza | journal = Revue Scientifique et Technique | volume = 28 | issue = 1 | pages = 19–38 | date = April 2009 | pmid = 19618616 | doi = 10.20506/rst.28.1.1856 | doi-access = }}</ref> Classification of a virus strain as either LPAI or HPAI is based on the severity of symptoms in domestic [[Chicken|chickens]] and does not predict severity of symptoms in other species. Chickens infected with LPAI display mild symptoms or are [[asymptomatic]], whereas HPAI causes serious breathing difficulties, significant drop in egg production, and sudden death.<ref name=":132">{{Cite web |last=CDC |date=2022-06-14 |title=Avian Influenza in Birds |url=https://www.cdc.gov/flu/avianflu/avian-in-birds.htm |access-date=2024-05-06 |website=Centers for Disease Control and Prevention |language=en-us}}</ref> |
|||
Since 2006, the [[World Organisation for Animal Health|World Organization for Animal Health]] requires all detections of LPAI H5 and H7 subtypes to be reported because of their potential to mutate into highly pathogenic strains.<ref>{{Cite web |date=October 2013 |title=National H5/H7 Avian Influenza surveillance plan |url=https://www.aphis.usda.gov/media/document/1295/file |website=United States Department of Agriculture |publisher=Animal Plant Health Inspection Service}}</ref> |
|||
;H5N2 |
|||
{{main|Influenza A virus subtype H5N2}} |
|||
:Japan's Health Ministry said January 2006 that poultry farm workers in Ibaraki prefecture may have been exposed to H5N2 in 2005.<ref>[http://www.cbsnews.com/stories/2006/01/10/health/main1195099.shtml CBS News] article ''Dozens In Japan May Have Mild Bird Flu'' January 2006.</ref> The H5N2 antibody titers of paired sera of 13 subjects increased fourfold or more.<ref>{{cite journal | vauthors = Ogata T, Yamazaki Y, Okabe N, Nakamura Y, Tashiro M, Nagata N, Itamura S, Yasui Y, Nakashima K, Doi M, Izumi Y, Fujieda T, Yamato S, Kawada Y | title = Human H5N2 avian influenza infection in Japan and the factors associated with high H5N2-neutralizing antibody titer | journal = Journal of Epidemiology | volume = 18 | issue = 4 | pages = 160–6 | date = July 2008 | pmid = 18603824 | pmc = 4771585 | doi = 10.2188/jea.JE2007446 | url = http://www.jstage.jst.go.jp/article/jea/18/4/160/_pdf | format = PDF }}</ref> |
|||
==== Pigs ==== |
|||
;H5N9 |
|||
{{Main|Swine influenza}} |
|||
{{anchor|H5N9}} |
|||
Signs of swine flu in pigs can include fever, depression, coughing (barking), discharge from the nose or eyes, sneezing, breathing difficulties, eye redness or inflammation, and going off feed. Some pigs infected with influenza, however, may show no signs of illness at all. Swine flu subtypes are principally H1N1, H1N2, and H3N2;<ref>{{Cite web |date=2017-06-15 |title=Factsheet on swine influenza in humans and pigs |url=https://www.ecdc.europa.eu/en/swine-influenza/factsheet |access-date=2024-06-25 |website=European Centre for Disease Prevention and Control |language=en}}</ref> it is spread either through close contact between animals or by the movement of contaminated equipment between farms.<ref>{{Cite web |date=2018-10-03 |title=Key Facts about Swine Influenza (Swine Flu) in Pigs {{!}} CDC |url=https://www.cdc.gov/flu/swineflu/keyfacts_pigs.htm |access-date=2024-06-25 |website=Centers for Disease Control and Prevention |language=en-us}}</ref> Humans who are in close contact with pigs can sometimes become infected.<ref>{{Cite web |date=30 March 2024 |title=2023: outbreaks of swine influenza |url=https://www.who.int/news/item/30-03-2024-2023--outbreaks-of-swine-influenza |access-date=2024-06-25 |website=World Health Organization |language=en}}</ref> |
|||
:A highly pathogenic strain of H5N9 caused a minor [[flu]] outbreak in 1966 in [[Ontario]] and [[Manitoba]], [[Canada]] in [[turkey (bird)|turkeys]].<ref>[http://www.who.int/csr/don/2004_03_02/en/ WHO]</ref> |
|||
==== Horses ==== |
|||
;H7N2 |
|||
{{Main|Equine influenza}} |
|||
{{main|Influenza A virus subtype H7N2}} |
|||
Equine influenza can affect horses, donkeys, and mules;<ref>{{Cite web |title=Equine influenza |url=https://www.woah.org/en/disease/equine-influenza-2/ |access-date=2024-06-25 |website=WOAH - World Organisation for Animal Health |language=en-GB}}</ref> it has a very high rate of transmission among horses, and a relatively short [[Incubation period|incubation time]] of one to three days.<ref name=":04">{{Cite web |title=Equine Influenza: Respiratory Diseases of Horses: Merck Veterinary Manual |url=http://www.merckvetmanual.com/mvm/respiratory_system/respiratory_diseases_of_horses/equine_influenza.html |url-status=dead |archive-url=https://web.archive.org/web/20161115191119/http://www.merckvetmanual.com/mvm/respiratory_system/respiratory_diseases_of_horses/equine_influenza.html |archive-date=2016-11-15 |access-date=2016-12-04 |website=www.merckvetmanual.com}}</ref> Clinical signs of equine influenza include fever, nasal discharge, have a dry, hacking cough, depression, loss of appetite and weakness.<ref name=":04" /> EI is caused by two subtypes of influenza A viruses: H7N7 and H3N8, which have evolved from avian influenza A viruses.<ref>{{Cite web |last=CDC |date=2023-05-05 |title=Horse Flu |url=https://www.cdc.gov/flu/other/horse-flu-faq.htm |access-date=2024-06-25 |website=Centers for Disease Control and Prevention |language=en-us}}</ref> |
|||
:One person in New York in 2003 and one person in [[Virginia]] in 2002 were found to have serologic evidence of infection with H7N2. Both fully recovered.<ref name=CDCAvFlu/> |
|||
==== Dogs ==== |
|||
;H7N3 |
|||
{{Main|Canine influenza}} |
|||
{{main|Influenza A virus subtype H7N3}} |
|||
Most animals infected with canine influenza A will show symptoms such as coughing, runny nose, fever, lethargy, eye discharge, and a reduced appetite lasting anywhere from 2–3 weeks.<ref name=":6" /> There are two different influenza A dog flu viruses: one is an H3N8 virus and the other is an H3N2 virus.<ref name=":6">{{Cite web |date=2023-08-29 |title=Key Facts about Canine Influenza (Dog Flu) {{!}} Seasonal Influenza (Flu) {{!}} CDC |url=https://www.cdc.gov/flu/other/canine-flu/keyfacts.html |access-date=2024-06-25 |website=Centers for Disease Protection and Control |language=en-us}}</ref> The H3N8 strain has evolved from an equine influenza avian virus which has adapted to sustained transmission among dogs. The H3N2 strain is derived from an avian influenza which jumped to dogs in 2004 in either Korea or China.<ref name=":6" /> It is likely that the virus persists in both animal shelters and kennels, as well as in farms where dogs are raised for meat production.<ref>{{cite journal | vauthors = Wasik BR, Voorhees IE, Parrish CR | title = Canine and Feline Influenza | journal = Cold Spring Harbor Perspectives in Medicine | volume = 11 | issue = 1 | pages = a038562 | date = January 2021 | pmid = 31871238 | pmc = 7778219 | doi = 10.1101/cshperspect.a038562 }}</ref> |
|||
:In North America, the presence of avian influenza strain H7N3 was confirmed at several poultry farms in British Columbia in February 2004. As of April 2004, 18 farms had been quarantined to halt the spread of the virus. Two cases of humans with avian influenza have been confirmed in that region. "Symptoms included conjunctivitis and mild influenza-like illness."<ref name=Tweed/> Both fully recovered. |
|||
==== Bats ==== |
|||
;H7N7 |
|||
{{Main|Bat influenza}} |
|||
{{main|Influenza A virus subtype H7N7}} |
|||
The first bat flu virus, IAV(H17N10), was first discovered in 2009 in [[Little yellow-shouldered bat|little yellow-shouldered bats]] (''Sturnira lilium'') in [[Guatemala]].<ref name="swwa">{{cite web |title=Bat Influenza (Flu) |url=https://www.cdc.gov/flu/other/bat-flu.html |access-date=30 June 2020 |website=cdc.gov}}</ref> In 2012 a second bat influenza A virus IAV(H18N11) was discovered in [[Flat-faced fruit-eating bat|flat-faced fruit-eating bats]] (''Artibeus planirostris'') from [[Peru]].<ref>{{cite web |title=Characterization of bat influenza viruses |url=https://www.uniklinik-freiburg.de/virologie-en/research/research-teams/martin-schwemmle-team/bat-influenza-viruses.html |access-date=30 June 2020 |website=uniklinik-freiburg.de}}</ref><ref>{{cite journal |date=2013 |title=New flu virus found in bats |url=https://www.nature.com/articles/503169e |journal=Nature |volume=503 |issue=7475 |page=169 |doi=10.1038/503169e |access-date=30 June 2020}}</ref><ref name="Schwemmle">{{cite journal | vauthors = Ciminski K, Pfaff F, Beer M, Schwemmle M | title = Bats reveal the true power of influenza A virus adaptability | journal = PLOS Pathogens | volume = 16 | issue = 4 | pages = e1008384 | date = April 2020 | pmid = 32298389 | pmc = 7161946 | doi = 10.1371/journal.ppat.1008384 | doi-access = free }}</ref> Bat influenza viruses have been found to be poorly adapted to non-bat species.<ref>{{cite journal | vauthors = Ciminski K, Ran W, Gorka M, Lee J, Malmlov A, Schinköthe J, Eckley M, Murrieta RA, Aboellail TA, Campbell CL, Ebel GD, Ma J, Pohlmann A, Franzke K, Ulrich R, Hoffmann D, García-Sastre A, Ma W, Schountz T, Beer M, Schwemmle M | title = Bat influenza viruses transmit among bats but are poorly adapted to non-bat species | journal = Nature Microbiology | volume = 4 | issue = 12 | pages = 2298–2309 | date = December 2019 | pmid = 31527796 | pmc = 7758811 | doi = 10.1038/s41564-019-0556-9 | s2cid = 202580293 }}</ref> |
|||
:H7N7 has unusual zoonotic potential. In 2003 in the Netherlands, 89 people were confirmed to have H7N7 influenza virus infection following an outbreak in poultry on several farms. One death was recorded. |
|||
== Research == |
|||
;H7N9 |
|||
Influenza research includes efforts to understand how influenza viruses enter hosts, the relationship between influenza viruses and bacteria, how influenza symptoms progress, and why some influenza viruses are deadlier than others.<ref name="niaid">{{cite web |date=13 March 2017 |title=Influenza Basic Research |url=https://www.niaid.nih.gov/diseases-conditions/influenza-basic-research |url-status=live |archive-url=https://web.archive.org/web/20240610052845/https://www.niaid.nih.gov/diseases-conditions/influenza-basic-research |archive-date=10 June 2024 |access-date=24 March 2021 |publisher=National Institute of Allergy and Infectious Diseases}}</ref> Past pandemics, and especially the 1918 pandemic, are the subject of much research to understand and prevent flu pandemics.<ref name="potter">{{cite journal | vauthors = Potter CW | title = A history of influenza | journal = Journal of Applied Microbiology | volume = 91 | issue = 4 | pages = 572–579 | date = October 2001 | pmid = 11576290 | doi = 10.1046/j.1365-2672.2001.01492.x | s2cid = 26392163 }}</ref><ref>{{cite journal | vauthors = Taubenberger JK, Baltimore D, Doherty PC, Markel H, Morens DM, Webster RG, Wilson IA | title = Reconstruction of the 1918 influenza virus: unexpected rewards from the past | journal = mBio | volume = 3 | issue = 5 | date = November 2012 | pmid = 22967978 | pmc = 3448162 | doi = 10.1128/mBio.00201-12 }}</ref> |
|||
{{main|Influenza A virus subtype H7N9}} |
|||
:On 2 April 2013, the [[Centre for Health Protection]] (CHP) of the Department of Health of Hong Kong confirmed four more cases in [[Jiangsu]] province in addition to the three cases initially reported on 31 March 2013.<ref>{{cite news | last = Schnirring | first = Lisa | name-list-format = vanc |title=China reports 4 more H7N9 infections | url = http://www.cidrap.umn.edu/cidrap/content/influenza/avianflu/news/apr0213china_(2)br.html | newspaper = CIDRAP News | date = 2 April 2013 }}</ref> This virus also has the greatest potential for an influenza pandemic among all of the Influenza A subtypes.<ref name="cdc.gov">{{cite web|title=Avian Influenza A (H7N9) Virus {{!}} Avian Influenza (Flu)|url=https://www.cdc.gov/flu/avianflu/h7n9-virus.htm|website=www.cdc.gov|accessdate=24 February 2017|language=en-us}}</ref> |
|||
The [[World Health Organization]] has published a Research Agenda with five streams:<ref name=":05">{{Cite web |date=2017 |title=WHO public health research agenda for influenza: 2017 update |url=https://www.who.int/initiatives/public-health-research-agenda-for-influenza |access-date=2024-06-28 |website=World Health Organization |place=Geneva |language=en}}</ref> |
|||
;H9N2 |
|||
{{main|Influenza A virus subtype H9N2}} |
|||
:Low pathogenic avian influenza A (H9N2) infection was confirmed in 1999, in China and Hong Kong in two children, and in 2003 in Hong Kong in one child. All three fully recovered.<ref name=CDCAvFlu>[https://www.cdc.gov/flu/avian/gen-info/avian-flu-humans.htm CDC] ''Avian Influenza Infection in Humans''</ref> |
|||
* Stream 1. Reducing the risk of emergence of pandemic influenza. This stream is entirely focused on preventing and limiting pandemic influenza; this includes research into what characteristics make a [[Strain (biology)|strain]] either mild or deadly, worldwide surveillance of influenza A viruses with pandemic potential, and the prevention and management of potentially zoonotic influenza in domestic and farmed animals.<ref name=":05" /> |
|||
;H10N7 |
|||
* Stream 2. Limiting the spread of pandemic, zoonotic and seasonal epidemic influenza. This is more broadly targeted at both pandemic and seasonal influenza, looking at the transmission of the virus between people and the ways in which it can spread globally, as well as the environmental and social factors which affect transmission.<ref name=":05" /> |
|||
{{main|Influenza A virus subtype H10N7}} |
|||
* Stream 3. Minimizing the impact of pandemic, zoonotic, and seasonal epidemic influenza. This is principally concerned with vaccination - improving the effectiveness of vaccines, vaccine technology, as well as the speed with which an effective vaccine can be developed and ways in which vaccines can be manufactured and delivered worldwide.<ref name=":05" /> |
|||
:In 2004 in [[Egypt]], H10N7 was reported for the first time in humans. It caused illness in two infants in Egypt. One child’s father was a poultry merchant.<ref>[http://www3.niaid.nih.gov/news/focuson/flu/illustrations/timeline/timeline.htm niaid.nih.gov] {{webarchive|url=https://web.archive.org/web/20051226195643/http://www3.niaid.nih.gov/news/focuson/flu/illustrations/timeline/timeline.htm |date=26 December 2005 }} Timeline of Human Flu Pandemics</ref> |
|||
* Stream 4. Optimizing the treatment of patients. This stream aims to reduce the impact of influenza by looking at methods of treatment, vulnerable groups, genetic predispositions, the interaction of influenza infection with other diseases, and influenza [[Sequela|sequelae]].<ref name=":05" /> |
|||
* Stream 5. Promoting the development and application of modern public health tools.<ref name=":05" /> Aiming to improve the ways in which public policy can combat influenza; this includes the introduction of new technologies, epidemic and pandemic [[Mathematical modelling of infectious diseases|modelling]], and the communication of accurate and trustworthy information to the public.<ref name=":05" /> |
|||
===Evolution=== |
|||
Taubenberger says: |
|||
:"All influenza A pandemics since [the Spanish flu pandemic], and indeed almost all cases of influenza A worldwide (excepting human infections from avian viruses such as H5N1 and H7N7), have been caused by descendants of the 1918 virus, including "drifted" H1N1 viruses and reassorted H2N2 and H3N2 viruses. The latter are composed of key genes from the 1918 virus, updated by subsequently incorporated avian influenza genes that code for novel surface proteins, making the 1918 virus indeed the "mother" of all pandemics."<ref>{{cite journal | vauthors = Taubenberger JK, Morens DM | title = 1918 Influenza: the mother of all pandemics | journal = Emerging Infectious Diseases | volume = 12 | issue = 1 | pages = 15–22 | date = January 2006 | pmid = 16494711 | pmc = 3291398 | doi = 10.3201/eid1201.050979 | df = dmy-all }}</ref> |
|||
Researchers from the [[National Institutes of Health]] used data from the [[Influenza Genome Sequencing Project]] and concluded that during the ten-year period examined, most of the time the hemagglutinin gene in H3N2 showed no significant excess of mutations in the antigenic regions while an increasing variety of strains accumulated. This resulted in one of the variants eventually achieving higher fitness, becoming dominant, and in a brief interval of rapid [[evolution]], rapidly sweeping through the population and eliminating most other variants.<ref>[https://www.sciencedaily.com/releases/2006/10/061026185115.htm Science Daily] article ''New Study Has Important Implications For Flu Surveillance'' published 27 October 2006</ref> |
|||
In the short-term evolution of influenza A virus, a 2006 study found that stochastic, or random, processes are key factors.<ref name="pmid17140286">{{cite journal | vauthors = Nelson MI, Simonsen L, Viboud C, Miller MA, Taylor J, George KS, Griesemer SB, Ghedin E, Ghedi E, Sengamalay NA, Spiro DJ, Volkov I, Grenfell BT, Lipman DJ, Taubenberger JK, Holmes EC | title = Stochastic processes are key determinants of short-term evolution in influenza a virus | journal = PLOS Pathogens | volume = 2 | issue = 12 | pages = e125 | date = December 2006 | pmid = 17140286 | pmc = 1665651 | doi = 10.1371/journal.ppat.0020125 }}</ref> Influenza A virus HA antigenic evolution appears to be characterized more by punctuated, sporadic jumps as opposed to a constant rate of antigenic change.<ref>{{cite journal | vauthors = Smith DJ, Lapedes AS, de Jong JC, Bestebroer TM, Rimmelzwaan GF, Osterhaus AD, Fouchier RA | title = Mapping the antigenic and genetic evolution of influenza virus | journal = Science | volume = 305 | issue = 5682 | pages = 371–6 | date = July 2004 | pmid = 15218094 | doi = 10.1126/science.1097211 | bibcode = 2004Sci...305..371S | url = https://semanticscholar.org/paper/f66a00d2bc5c04dd68f672659f97eff8e7084dc1 }}</ref> Using phylogenetic analysis of 413 complete genomes of human influenza A viruses that were collected throughout the state of New York, the authors of Nelson et al. 2006 were able to show that genetic diversity, and not antigenic drift, shaped the short-term evolution of influenza A via random migration and reassortment. The evolution of these viruses is dominated more by the random importation of genetically different viral strains from other geographic locations and less by natural selection. Within a given season, adaptive evolution is infrequent and had an overall weak effect as evidenced from the data gathered from the 413 genomes. Phylogenetic analysis revealed the different strains were derived from newly imported genetic material as opposed to isolates that had been circulating in New York in previous seasons. Therefore, the gene flow in and out of this population, and not natural selection, was more important in the short term. |
|||
==Other animals== |
|||
:''See [[H5N1]] for the current [[epizootic]] (an epidemic in nonhumans) and panzootic (a disease affecting animals of many species especially over a wide area) of H5N1 influenza'' |
|||
;Avian influenza |
|||
{{main|Avian influenza}} |
|||
[[Fowl]] act as natural [[asymptomatic carrier]]s of influenza A viruses. Prior to the current H5N1 epizootic, strains of influenza A virus had been demonstrated to be transmitted from wild fowl to only birds, pigs, horses, [[Pinniped|seals]], whales and humans; and only between humans and pigs and between humans and domestic fowl; and not other pathways such as domestic fowl to horse.<ref>{{harvnb|Mahmoud|2005|p=[http://www.nap.edu/books/0309095042/html/30.html 30]}}</ref> |
|||
Wild aquatic birds are the natural hosts for a large variety of influenza A viruses. Occasionally, viruses are transmitted from these birds to other species and may then cause devastating outbreaks in domestic poultry or give rise to human influenza pandemics.<ref name="sobrino6"/><ref name="Kawaoka"/> |
|||
H5N1 has been shown to be transmitted to tigers, leopards, and domestic cats that were fed uncooked domestic fowl (chickens) with the virus. [[Influenza A virus subtype H3N8|H3N8]] viruses from horses have crossed over and caused outbreaks in dogs. Laboratory mice have been infected successfully with a variety of avian flu genotypes.<ref>{{harvnb|Mahmoud|2005|p=[http://www.nap.edu/books/0309095042/html/82.html 82]}}<br /> "Interestingly, recombinant influenza viruses containing the 1918 HA and NA and up to three additional genes derived from the 1918 virus (the other genes being derived from the A/WSN/33 virus) were all highly virulent in mice (Tumpey et al., 2004). Furthermore, expression microarray analysis performed on whole lung tissue of mice infected with the 1918 HA/ NA recombinant showed increased upregulation of genes involved in apoptosis, tissue injury, and oxidative damage (Kash et al., 2004). These findings were unusual because the viruses with the 1918 genes had not been adapted to mice. The completion of the sequence of the entire genome of the 1918 virus and the reconstruction and characterization of viruses with 1918 genes under appropriate biosafety conditions will shed more light on these findings and should allow a definitive examination of this explanation. Antigenic analysis of recombinant viruses possessing the 1918 HA and NA by hemagglutination inhibition tests using ferret and chicken antisera suggested a close relationship with the A/swine/Iowa/30 virus and H1N1 viruses isolated in the 1930s (Tumpey et al., 2004), further supporting data of Shope from the 1930s (Shope, 1936). Interestingly, when mice were immunized with different H1N1 virus strains, challenge studies using the 1918-like viruses revealed partial protection by this treatment, suggesting that current vaccination strategies are adequate against a 1918-like virus (Tumpey et al., 2004)."</ref> |
|||
Influenza A viruses spread in the air and in [[manure]], and survives longer in cold weather. It can also be transmitted by contaminated feed, water, equipment and clothing; however, there is no evidence the virus can survive in well-cooked meat. Symptoms in animals vary, but virulent strains can cause death within a few days. |
|||
"Highly pathogenic avian influenza virus is on every top ten list available for potential agricultural bioweapon agents".<ref>{{harvnb|Mahmoud|2005|p=[http://www.nap.edu/books/0309095042/html/285.html 285]}}<br />"As of October 2001, the potential for use of infectious agents, such as anthrax, as weapons has been firmly established. It has been suggested that attacks on a nation’s agriculture might be a preferred form of terrorism or economic disruption that would not have the attendant stigma of infecting and causing disease in humans. Highly pathogenic avian influenza virus is on every top ten list available for potential agricultural bioweapon agents, generally following foot and mouth disease virus and Newcastle disease virus at or near the top of the list. Rapid detection techniques for bioweapon agents are a critical need for the first-responder community, on a par with vaccine and antiviral development in preventing spread of disease."</ref> |
|||
Avian influenza viruses that the [[OIE]] and others test for to control poultry disease include: [[H5N1]], [[H7N2]], [[H1N7]], [[H7N3]], [[H13N6]], [[H5N9]], H11N6, [[H3N8]], [[H9N2]], [[H5N2]], H4N8, [[H10N7]], [[H2N2]], H8N4, H14N5, H6N5, H12N5 and others. |
|||
;Known outbreaks of highly pathogenic flu in poultry 1959–2003<ref>{{cite web |url=http://www.who.int/csr/don/2004_03_02/en/ |title=Avian influenza A(H5N1)- update 31: Situation (poultry) in Asia: need for a long-term response, comparison with previous outbreaks |year=2004 |work=Epidemic and Pandemic Alert and Response (EPR) |publisher=WHO }}<br />Known outbreaks of highly pathogenic flu in poultry 1959–2003.</ref> |
|||
{| class="wikitable sortable" |
|||
|- |
|||
!Year |
|||
!Area |
|||
!Affected |
|||
!Subtype |
|||
|- |
|||
| 1959 |
|||
| Scotland |
|||
| Chicken |
|||
| [[H5N1]] |
|||
|- |
|||
| 1963 |
|||
| England |
|||
| Turkey |
|||
| [[H7N3]] |
|||
|- |
|||
| 1966 |
|||
| Ontario (Canada) |
|||
| Turkey |
|||
| [[H5N9]] |
|||
|- |
|||
| 1976 |
|||
| Victoria (Australia) |
|||
| Chicken |
|||
| [[H7N7]] |
|||
|- |
|||
| 1979 |
|||
| Germany |
|||
| Chicken |
|||
| H7N7 |
|||
|- |
|||
| 1979 |
|||
| England |
|||
| Turkey |
|||
| H7N7 |
|||
|- |
|||
| 1983 |
|||
| Pennsylvania (US)* |
|||
| Chicken, turkey |
|||
| [[H5N2]] |
|||
|- |
|||
| 1983 |
|||
| Ireland |
|||
| Turkey |
|||
| [[H5N8]] |
|||
|- |
|||
| 1985 |
|||
| Victoria (Australia) |
|||
| Chicken |
|||
| H7N7 |
|||
|- |
|||
| 1991 |
|||
| England |
|||
| Turkey |
|||
| H5N1 |
|||
|- |
|||
| 1992 |
|||
| Victoria (Australia) |
|||
| Chicken |
|||
| H7N3 |
|||
|- |
|||
| 1994 |
|||
| Queensland (Australia) |
|||
| Chicken |
|||
| H7N3 |
|||
|- |
|||
| 1994 |
|||
| Mexico* |
|||
| Chicken |
|||
| H5N2 |
|||
|- |
|||
| 1994 |
|||
| Pakistan* |
|||
| Chicken |
|||
| H7N3 |
|||
|- |
|||
| 1997 |
|||
| New South Wales (Australia) |
|||
| Chicken |
|||
| [[H7N4]] |
|||
|- |
|||
| 1997 |
|||
| Hong Kong (China)* |
|||
| Chicken |
|||
| H5N1 |
|||
|- |
|||
| 1997 |
|||
| Italy |
|||
| Chicken |
|||
| H5N2 |
|||
|- |
|||
| 1999 |
|||
| Italy* |
|||
| Turkey |
|||
| [[H7N1]] |
|||
|- |
|||
| 2002 |
|||
| Hong Kong (China) |
|||
| Chicken |
|||
| H5N1 |
|||
|- |
|||
| 2002 |
|||
| Chile |
|||
| Chicken |
|||
| H7N3 |
|||
|- |
|||
| 2003 |
|||
| Netherlands* |
|||
| Chicken |
|||
| H7N7 |
|||
|} |
|||
<small>'' *Outbreaks with significant spread to numerous farms, resulting in great economic losses. Most other outbreaks involved little or no spread from the initially infected farms.'' </small> |
|||
1979: "More than 400 harbor seals, most of them immature, died along the New England coast between December 1979 and October 1980 of acute pneumonia associated with influenza virus, A/Seal/Mass/1/180 (H7N7)."<ref>{{cite journal | vauthors = Geraci JR, St Aubin DJ, Barker IK, Webster RG, Hinshaw VS, Bean WJ, Ruhnke HL, Prescott JH, Early G, Baker AS, Madoff S, Schooley RT | title = Mass mortality of harbor seals: pneumonia associated with influenza A virus | journal = Science | volume = 215 | issue = 4536 | pages = 1129–31 | date = February 1982 | pmid = 7063847 | doi = 10.1126/science.7063847 | quote = More than 400 harbor seals, most of them immature, died along the New England coast between December 1979 and October 1980 of acute pneumonia associated with influenza virus, A/Seal/Mass/1/180 (H7N7). The virus has avian characteristics, replicates principally in mammals, and causes mild respiratory disease in experimentally infected seals. Concurrent infection with a previously undescribed mycoplasma or adverse environmental conditions may have triggered the epizootic. The similarities between this epizootic and other seal mortalities in the past suggest that these events may be linked by common biological and environmental factors. | bibcode = 1982Sci...215.1129G }}</ref> |
|||
1995: "[V]accinated birds can develop asymptomatic infections that allow virus to spread, mutate, and recombine (ProMED-mail, 2004j). Intensive surveillance is required to detect these “silent epidemics” in time to curtail them. In Mexico, for example, mass vaccination of chickens against epidemic H5N2 influenza in 1995 has had to continue in order to control a persistent and evolving virus (Lee et al., 2004)."<ref>{{harvnb|Mahmoud|2005|p=[http://www.nap.edu/books/0309095042/html/15.html 15]}}<br />"Unlike most other affected countries, Indonesia also instituted mass vaccination of healthy domestic birds against H5N1, followed by routine vaccination (China has a similar policy; other Asian countries are considering it [ProMED-mail, 2004j]) (Soebandrio, 2004). This is a risky strategy, because vaccinated birds can develop asymptomatic infections that allow virus to spread, mutate, and recombine (ProMED-mail, 2004j). Intensive surveillance is required to detect these "silent epidemics" in time to curtail them. In Mexico, for example, mass vaccination of chickens against epidemic H5N2 influenza in 1995 has had to continue in order to control a persistent and evolving virus (Lee et al., 2004)."</ref> |
|||
1997: "Influenza A viruses normally seen in one species sometimes can cross over and cause illness in another species. For example, until 1997, only H1N1 viruses circulated widely in the US pig population. However, in 1997, H3N2 viruses from humans were introduced into the pig population and caused widespread disease among pigs. Most recently, H3N8 viruses from horses have crossed over and caused outbreaks in dogs."<ref>[https://www.cdc.gov/flu/avian/gen-info/transmission.htm CDC] Centers for Disease Control and Prevention – ''Transmission of Influenza A Viruses Between Animals and People''</ref> |
|||
2000: "In California, poultry producers kept their knowledge of a recent H6N2 avian influenza outbreak to themselves due to their fear of public rejection of poultry products; meanwhile, the disease spread across the western United States and has since become endemic."<ref>{{harvnb|Mahmoud|2005|p=[http://www.nap.edu/books/0309095042/html/27.html 27]}}</ref> |
|||
2003: In Netherlands H7N7 influenza virus infection broke out in poultry on several farms.<ref>[http://news.bbc.co.uk/1/hi/programmes/panorama/4412932.stm BBC News] ''Early bird flu warning for Dutch'' – 6 November 2005</ref> |
|||
2004: In North America, the presence of avian influenza strain H7N3 was confirmed at several poultry farms in [[British Columbia]] in February 2004. As of April 2004, 18 farms had been quarantined to halt the spread of the virus.<ref name=Tweed>{{cite journal | vauthors = Tweed SA, Skowronski DM, David ST, Larder A, Petric M, Lees W, Li Y, Katz J, Krajden M, Tellier R, Halpert C, Hirst M, Astell C, Lawrence D, Mak A | title = Human illness from avian influenza H7N3, British Columbia | journal = Emerging Infectious Diseases | volume = 10 | issue = 12 | pages = 2196–9 | date = December 2004 | pmid = 15663860 | pmc = 3323407 | doi = 10.3201/eid1012.040961 }}</ref> |
|||
2005: Tens of millions of birds died of H5N1 influenza and hundreds of millions of birds were culled to protect humans from H5N1. H5N1 is endemic in birds in southeast Asia and represents a long-term pandemic threat. |
|||
2006: H5N1 spreads across the globe, killing hundreds of millions of birds and over 100 people, and causing a significant [[H5N1 impact]] from both actual deaths and predicted possible deaths. |
|||
;Swine flu |
|||
{{main|Swine influenza}} |
|||
:Swine influenza (or "pig influenza") refers to a subset of Orthomyxoviridae that create influenza and are endemic in pigs. The species of Orthomyxoviridae that can cause flu in pigs are influenza A virus and [[Influenzavirus C|influenza C virus]], but not all genotypes of these two species infect pigs. The known subtypes of influenza A virus that create influenza and are endemic in pigs are H1N1, H1N2, [[Influenza A virus subtype H3N1|H3N1]] and H3N2. |
|||
;Horse flu |
|||
{{main|Equine influenza}} |
|||
:Horse flu (or "equine influenza") refers to varieties of influenza A virus that affect horses. Horse flu viruses were only isolated in 1956. The two main types of virus are called equine-1 (H7N7), which commonly affects horse heart muscle, and equine-2 (H3N8), which is usually more severe. |
|||
;Dog flu |
|||
{{main|Canine influenza}} |
|||
:Dog flu (or "canine influenza") refers to varieties of influenza A virus that affect dogs. The equine influenza virus H3N8 was found to infect and kill – with respiratory illness – greyhound race dogs at a Florida racetrack in January 2004. |
|||
;Bat flu |
|||
{{main|Bat influenza}} |
|||
:Bat flu (or "Bat influenza") refers to the H17N10 and H18N11 influenza A virus strains that were discovered in Central and South American fruit bats as well as a H9N2 virus isolated from the Egyptian fruit bat.<ref>{{cite journal | vauthors = Kandeil A, Gomaa MR, Shehata MM, El Taweel AN, Mahmoud SH, Bagato O | title = Isolation and Characterization of a Distinct Influenza A Virus from Egyptian Bats | journal = Journal of Virology | volume = 93 | issue = 2 | pages = e01059-18 | date = January 2019 | pmid = 30381492 | pmc = 6321940 | doi = 10.1128/JVI.01059-18 }}</ref> Until now it is unclear whether these bat-derived viruses are circulating in any non-bat species and whether they pose a zoonotic threat. Initial characterization of the H18N11 subtype, however, suggests that this bat influenza virus is not well adapted to any other species than bats.<ref>{{cite journal | vauthors = Ciminski K, Ran W, Gorka M, Lee J, Schinköthe J, Eckley M, Murrieta MA, Aboellail TA, Campbell CL, Ebel GD, Ma J, Pohlmann A, Franzke K, Ulrich R, Hoffmann D, Garcia-Sastre A, Ma W, Schountz T, Beer M, Schwemmle M | title = Bat influenza viruses transmit among bats but are poorly adapted to non-bat species | journal = Nature Microbiology | volume = 4 | issue = 12 | pages = 2298–2309 | pmid = 31527796 | doi = 10.1038/s41564-019-0556-9 | year = 2019 }}</ref> |
|||
;H3N8 |
|||
{{main|Influenza A virus subtype H3N8}} |
|||
:H3N8 is now endemic in birds, horses and dogs. |
|||
== See also == |
== See also == |
||
* [[FI6 (antibody)]] |
|||
* [[Influenza vaccine]] |
* [[Influenza vaccine]] |
||
* [[Veterinary virology]] |
* [[Veterinary virology]] |
||
== References == |
|||
==Notes== |
|||
{{Reflist|30em}} |
{{Reflist|30em}} |
||
== Further reading == |
== Further reading == |
||
;Official sources |
;Official sources |
||
{{ |
{{Further|H5N1}} |
||
* [https://www.cdc.gov/flu/ |
* [https://www.cdc.gov/flu/avianflu/ Information on Bird Flu] US [[Centers for Disease Control and Prevention]] (CDC) |
||
* [https://www.cdc.gov/flu/pandemic-resources/index.htm Pandemic Influenza] US CDC |
|||
* [https://web.archive.org/web/20041124215153/http://www.who.int/csr/disease/avian_influenza/avian_faqs/en/ Avian influenza] [[FAQ]] from the [[World Health Organization]] |
|||
* [https://web.archive.org/web/20060212152800/http://www.fao.org/ag/againfo/subjects/en/health/diseases-cards/special_avian.html Avian influenza information] from the [[Food and Agriculture Organization]] |
|||
* [http://www.pandemicflu.gov U.S. Government's avian influenza information website] |
|||
* [https://web.archive.org/web/20060223025537/http://www.ecdc.eu.int/ European Centre for Disease Prevention and Control] ([[ECDC]]) Stockholm, Sweden |
|||
;General information |
;General information |
||
{{ |
{{Further|Flu}} |
||
* [http://www.nature.com/nature/focus/avianflu/index.html Web focus: Warnings of a Flu Pandemic] ''[[Nature (journal)|Nature]]'' |
|||
* [https://web.archive.org/web/20060219134619/http://www.ndu.edu/ctnsp/Bird_flu.htm "The Bird Flu and You"] Full-color poster provided by the Center for Technology and National Security Policy at the [[National Defense University]], in collaboration with the National Security Health Policy Center |
|||
* [http://www.InfluenzaReport.com Influenza Report 2006] Online book. Research level quality information. Highly recommended. |
|||
* [http://www.nature.com/nature/focus/avianflu/index.html Special issue on avian flu] from ''[[Nature (journal)|Nature]]'' |
|||
* [http://www.nature.com/avianflu/index.html Nature Reports: Homepage: Avian Flu] |
* [http://www.nature.com/avianflu/index.html Nature Reports: Homepage: Avian Flu] |
||
* {{cite journal | vauthors = Beigel JH, Farrar J, Han AM, Hayden FG, Hyer R, de Jong MD, Lochindarat S, Nguyen TK, Nguyen TH, Tran TH, Nicoll A, Touch S, Yuen KY | title = Avian influenza A (H5N1) infection in humans | journal = The New England Journal of Medicine | volume = 353 | issue = 13 | pages = |
* {{cite journal | vauthors = Beigel JH, Farrar J, Han AM, Hayden FG, Hyer R, de Jong MD, Lochindarat S, Nguyen TK, Nguyen TH, Tran TH, Nicoll A, Touch S, Yuen KY | title = Avian influenza A (H5N1) infection in humans | journal = The New England Journal of Medicine | volume = 353 | issue = 13 | pages = 1374–1385 | date = September 2005 | pmid = 16192482 | doi = 10.1056/NEJMra052211 | citeseerx = 10.1.1.730.7890 }} |
||
* [https://digital.library.unt.edu/govdocs/crs/permalink/meta-crs-7927 Pandemic Influenza: Domestic Preparedness Efforts] Congressional Research Service Report on Pandemic Preparedness. |
* [https://digital.library.unt.edu/govdocs/crs/permalink/meta-crs-7927 Pandemic Influenza: Domestic Preparedness Efforts] Congressional Research Service Report on Pandemic Preparedness. |
||
* {{cite book |last=Mahmoud |title=The threat of pandemic influenza : are we ready? : workshop summary / prepared for Forum on Microbial Threats, Board on Global Health |editor=Stacey L. Knobler |editor2=Alison Mack |editor3-first=Adel |editor3-last=Mahmoud |editor4=Stanley M. Lemon |isbn=0-309-09504-2 |publisher=The National Academies Press |year=2005 |page=285 |quote=Highly pathogenic avian influenza virus is on every top ten list available for potential agricultural bioweapon agents}} |
|||
* [https://www.bbc.co.uk/health/conditions/birdflu1.shtml A guide to bird flu and its symptoms] from [[BBC]] Health |
|||
* {{cite book |last1=Mahmoud |first1=Adel A. F |author2=Institute of Medicine | last3 = Knobler | first3 = Stacey | last4 = Mack | first4 = Alison |title=The Threat of Pandemic Influenza: Are We Ready?: Workshop Summary |publisher=National Academies Press |location=Washington, D.C. |year=2005 |isbn=978-0-309-09504-4 |url=http://www.nap.edu/openbook.php?record_id=11150&page=R1}} |
|||
* [https://web.archive.org/web/20060215185754/http://3dscience.com/Avian_Flu_Bird_Flu_License_Free_Images.asp A Variety of Avian Flu Images and Pictures] |
|||
* {{harvnb|Mahmoud|2005|p=[http://www.nap.edu/books/0309095042/html/285.html 285]}} "Highly pathogenic avian influenza virus is on every top ten list available for potential agricultural bioweapon agents" |
|||
* {{cite book |last1=Mahmoud |first1=Adel A. F |author2=Institute of Medicine | last3 = Knobler | first3 = Stacey | last4 = Mack | first4 = Alison | name-list-format = vanc |title=The Threat of Pandemic Influenza: Are We Ready?: Workshop Summary |publisher=National Academies Press |location=Washington, D.C |year=2005 |isbn=978-0-309-09504-4 |url=http://www.nap.edu/openbook.php?record_id=11150&page=R1 |ref=harv }} |
|||
* [https://web.archive.org/web/20051123144004/http://www.healthpolitics.com/archives.asp?previous=bird_flu 'The Threat of Bird Flu': HealthPolitics.com] |
|||
* [https://web.archive.org/web/20060211143519/http://www.infectioncontroltoday.com/articles/531feat1.html Is a Global Flu Pandemic Imminent?] from ''Infection Control Today''. |
|||
* [http://www.ninthday.com/bird_flu.htm Bird Flu is a Real Pandemic Threat to Humans] by Leonard Crane, author of ''Ninth Day of Creation''. |
|||
* [http://www.lib.uiowa.edu/hardin/md/birdflu.html Links to Bird Flu pictures (Hardin MD/Univ of Iowa)] |
* [http://www.lib.uiowa.edu/hardin/md/birdflu.html Links to Bird Flu pictures (Hardin MD/Univ of Iowa)] |
||
* {{cite book | |
* {{cite book | vauthors = Kawaoka Y |title=Influenza Virology: Current Topics |publisher=Caister Academic Pr |year=2006 |isbn=978-1-904455-06-6 }} |
||
* {{cite book | |
* {{cite book | vauthors = Sobrino F, Mettenleiter T |title=Animal Viruses: Molecular Biology |publisher=Caister Academic Press |year=2008 |isbn=978-1-904455-22-6 }} |
||
== External links == |
|||
* [http://www.fludb.org Influenza Research Database] – Database of influenza genomic sequences and related information. |
|||
* [http://ec.europa.eu/health-eu/health_problems/avian_influenza/index_en.htm Health-EU portal] [[European Union]] response to influenza |
|||
{{Influenza}} |
{{Influenza}} |
||
{{Common cold}} |
{{Common cold}} |
||
{{Viral diseases}} |
{{Viral diseases}} |
||
{{Portal bar|Viruses}} |
{{Portal bar|Medicine|Viruses}} |
||
{{Taxonbar|from=Q834390}} |
{{Taxonbar|from=Q834390}} |
||
{{Authority control}} |
|||
{{DEFAULTSORT:Influenza A Virus}} |
{{DEFAULTSORT:Influenza A Virus}} |
||
[[Category:Influenza A virus| ]] |
|||
[[Category:Animal virology]] |
[[Category:Animal virology]] |
||
[[Category:Influenza A virus| ]] |
|||
[[Category:Zoonoses]] |
[[Category:Zoonoses]] |
||
Latest revision as of 20:17, 9 September 2024
| Influenza A virus | |
|---|---|
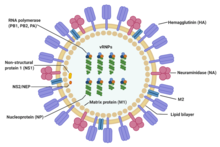
| |
| Structure of influenza A virus | |
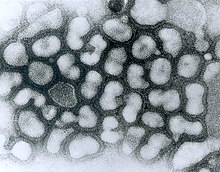
| |
| TEM micrograph of influenza A viruses | |
| Virus classification | |
| (unranked): | Virus |
| Realm: | Riboviria |
| Kingdom: | Orthornavirae |
| Phylum: | Negarnaviricota |
| Class: | Insthoviricetes |
| Order: | Articulavirales |
| Family: | Orthomyxoviridae |
| Genus: | Alphainfluenzavirus |
| Species: | Influenza A virus
|
Influenza A virus (IAV) is the only species of the genus Alphainfluenzavirus of the virus family Orthomyxoviridae.[1] It is a pathogen with strains that infect birds and some mammals, as well as causing seasonal flu in humans.[2] Mammals in which different strains of IAV circulate with sustained transmission are bats, pigs, horses and dogs; other mammals can occasionally become infected.[3][4]
IAV is an enveloped negative-sense RNA virus, with a segmented genome.[4] Through a combination of mutation and genetic reassortment the virus can evolve to acquire new characteristics, enabling it to evade host immunity and occasionally to jump from one species of host to another.[5][6]
Subtypes of IAV are defined by the combination of the antigenic H and N proteins in the viral envelope; for example, "H1N1" designates an IAV subtype that has a type-1 hemagglutinin (H) protein and a type-1 neuraminidase (N) protein.[7] Almost all possible combinations of H (1 thru 16) and N (1 thru 11) have been isolated from wild birds.[8] Further variations exist within the subtypes and can lead to very significant differences in the virus's ability to infect and cause disease, as well as to the severity of symptoms.[9][10]
Symptoms of human seasonal flu usually include fever, cough, sore throat, muscle aches, conjunctivitis and, in severe cases, breathing problems and pneumonia that may be fatal.[11][2] Humans can rarely become infected with strains of avian or swine influenza, usually as a result of close contact with infected animals; symptoms range from mild to severe including death.[12][13] Bird-adapted strains of the virus can be asymptomatic in some aquatic birds but lethal if they spread to other species, such as chickens.[14]
IAV disease in poultry can be can be prevented by vaccination, however biosecurity control measures are preferred.[15][16] In humans, seasonal influenza can be treated in its early stages with antiviral medicines.[17] A global network, the Global Influenza Surveillance and Response System (GISRS) monitors the spread of influenza with the aim to inform development of both seasonal and pandemic vaccines.[18] Several millions of specimens are tested by the GISRS network annually through a network of laboratories in 127 countries. As well as human viruses, GISRS monitors avian, swine, and other potentially zoonotic influenza viruses. IAV vaccines need to be reformulated regularly in order to keep up with changes in the virus.[19]
Virology
[edit]Classification
[edit]There are two methods of classification, one based on surface proteins (originally serotypes),[20] and the other based on its behavior, mainly the host animal.
Subtypes
[edit]
There are two antigenic proteins on the surface of the viral envelope, hemagglutinin and neuraminidase.[21] Different influenza virus genomes encode different hemagglutinin and neuraminidase proteins. Based on their serotype, there are 18 known types of hemagglutinin and 11 types of neuraminidase.[22][23] Subtypes of IAV are classified by their combination of H and N proteins. For example, "H5N1" designates an influenza A subtype that has a type-5 hemagglutinin (H) protein and a type-1 neuraminidase (N) protein.[22] Further variations exist within the subtypes and can lead to very significant differences in the virus's behavior.[24]
By definition, the subtyping scheme only takes into account the two outer proteins, not the at least 8 proteins internal to the virus.[25] Almost all possible combinations of H (1 thru 16) and N (1 thru 11) have been isolated from wild birds.[26] H17 and H18 have only been discovered in bats.[27]
Influenza virus nomenclature
[edit]Due to the high variability of the virus, subtyping is not sufficient to uniquely identify a strain of influenza A virus. To unambiguously describe a specific isolate of virus, researchers use the Influenza virus nomenclature,[28] which describes, among other things, the subtype, year, and place of collection. Some examples include:[29]
- A/Rio de Janeiro/62434/2021 (H3N2).[29]
- The starting A indicates that the virus is an influenza A virus.
- Rio de Janeiro indicates the place of collection. 62434 is a laboratory sequence number. 2021 (or just 21) indicates that the sample was collected in 2021. No species is mentioned so by default, the sample was collected from a human.
- (H3N2) indicates the subtype of the virus.
- A/swine/South Dakota/152B/2009 (H1N2).[29]
- This example shows an additional field before the place: swine. It indicates that the sample was collected from a pig.
- A/California/04/2009 A(H1N1)pdm09.[29]
- This example carries an unusual designation in the last part: instead of a usual (H1N1), it uses A(H1N1)pdm09. This was in order to distinguish the Pandemic H1N1/09 virus lineage from older H1N1 viruses.[29]
Structure and genetics
[edit]
Structure
[edit]The influenza A virus has a negative-sense, single-stranded, segmented RNA genome, enclosed in a lipid envelope. The virus particle (also called the virion) is 80–120 nanometers in diameter such that the smallest virions adopt an elliptical shape; larger virions have a filamentous shape.[30]
Core - The central core of the virion contains the viral RNA genome, which is made of eight separate segments.[31] The nucleoprotein (NP) coats the viral RNA to form a ribonucleoprotein that assumes a helical (spiral) configuration. Three large proteins (PB1, PB2, and PA), which are responsible for RNA transcription and replication, are bound to each segment of viral RNP.[31][32][33]
Capsid - The matrix protein M1 forms a layer between the nucleoprotein and the envelope, called the capsid.[31][32][33]
Envelope - The viral envelope consists of a lipid bilayer derived from the host cell. Two viral proteins; hemagglutinin (HA) and neuraminidase (NA), are inserted into the envelope and are exposed as spikes on the surface of the virion. Both proteins are antigenic; a host's immune system can react to them and produce antibodies in response. The M2 protein forms an ion channel in the envelope and is responsible for uncoating the virion once it has bound to a host cell.[31][32][33]
Genome
[edit]The table below presents a concise summary of the influenza genome and the principal functions of the proteins which are encoded. Segments are conventionally numbered from 1 to 8 in descending order of length.[34][35][36][37]
| RNA Segment | Length | Protein | Function |
| 1- PB2 | 2341 | PB2 (Polymerase Basic 2) | A component of the viral RNA polymerase.
PB2 also inhibits JAK1/STAT signaling to inhibit host innate immune response |
| 2- PB1 | 2341 | PB1 (Polymerase Basic 1) | A component of the viral RNA polymerase.
It also degrades the host cell’s mitochondrial antiviral signaling protein |
| PB1-F2 (Polymerase Basic 1-Frame 2) | An accessory protein of most IAVs. Not needed for virus replication and growth, it interferes with the host immune response. | ||
| 3- PA | 2233 | PA (Polymerase Acid) | A component of the viral RNA polymerase |
| PA-X | Arises from a ribosomal frameshift in the PA segment. Inhibits innate host immune responses, such as cytokine and interferon production. | ||
| 4- HA | 1775 | HA (Hemagglutinin) | Part of the viral envelope, a protein that binds the virion to host cells, enabling the virus’s RNA genetic material to invade it |
| 5- NP | 1565 | NP (Nucleoprotein) | The nucleoprotein associates with the viral RNA to form a ribonucleoprotein (RNP).
At the early stage of infection, the RNP binds to the host cell’s importin-α which transports it into the host cell nucleus, where the viral RNA is transcribed and replicated. At a later stage of infection, newly manufactured viral RNA segments assemble with the NP protein and polymerase (PB1, PB2 and PA) to form the core of a progeny virion |
| 6- NA | 1409 | NA (Neuraminidase) | Part of the viral envelope. NA enables the newly assembled virions to escape the host cell and go on to propagate the infection.
NA also facilitates the movement of infective virus particles through mucus, enabling them to reach host epithelial cells. |
| 7- M | 1027 | M1 (Matrix Protein 1) | Forms the capsid, which coats the viral nucleoproteins and supports the structure of the viral envelope.
M1 also assists with the function of the NEP protein. |
| M2 (Matrix Protein 2) | Forms a proton channel in the viral envelope, which is activated once a virion has bound to a host cell. This uncoats the virus, exposing its infective contents to the cytoplasm of the host cell | ||
| 8- NS | 890 | NS1 (non-structural protein 1) | Counteracts the host’s natural immune response and inhibits interferon production. |
| NEP (Nuclear Export Protein, formerly NS2 non-structural protein 2) | Cooperates with the M1 protein to mediate the export of viral RNA copies from nucleus into cytoplasm in the late stage of viral replication |
Three viral proteins - PB1, PB2, and PA - associate to form the RNA-dependent RNA polymerase (RdRp) which functions to transcribe and replicate the viral RNA.
Viral messenger RNA Transcription - The RdRp complex transcribes viral mRNAs by using a mechanism called cap-snatching. It consists in the hijacking and cleavage of host capped pre-mRNAs. Host cell mRNA is cleaved near the cap to yield a primer for the transcription of positive-sense viral mRNA using the negative-sense viral RNA as a template.[38] The host cell then transports the viral mRNA into the cytoplasm where ribosomes manufacture the viral proteins.[34][35][36][37]
Replication of the viral RNA -The replication of the influenza virus, unlike most other RNA viruses,[39] takes place in the nucleus and involves two steps. The RdRp first of all transcribes the negative-sense viral genome into a positive-sense complimentary RNA (cRNA), then the cRNAs are used as templates to transcribe new negative-sense vRNA copies. These are exported from the nucleus and assemble near the cell membrane to form the core of new virions.[34][35][36][37]
Epidemiology
[edit]Evolution and history
[edit]
The predominant natural reservoir of influenza viruses is thought to be wild waterfowl.[40] The subtypes of influenza A virus are estimated to have diverged 2,000 years ago. Influenza viruses A and B are estimated to have diverged from a single ancestor around 4,000 years ago, while the ancestor of influenza viruses A and B and the ancestor of influenza virus C are estimated to have diverged from a common ancestor around 8,000 years ago.[41]
Outbreaks of influenza-like disease can be found throughout recorded history. The first probable record is by Hippocrates in 142 BCE.[42] The historian Fujikawa listed 46 epidemics of flu-like illness in Japan between 862 and 1868.[43] In Europe and the Americas, a number of epidemics were recorded through the Middle Ages and up to the end of the 19th century.[42]

In 1918-1919 came the first flu pandemic of the 20th century, known generally as the "Spanish flu", which caused an estimated 20 to 50 million deaths worldwide. It is now known that this was caused by an immunologically novel H1N1 subtype of influenza A.[44] The next pandemic took place in 1957, the "Asian flu", which was caused by a H2N2 subtype of the virus in which the genome segments coding for HA and NA appeared to have derived from avian influenza strains by reassortment, while the remainder of the genome was descended from the 1918 virus.[45] The 1968 pandemic ("Hong Kong flu") was caused by a H3N2 subtype in which the NA segment was derived from the 1957 virus, while the HA segment had been reassorted from an avian strain of influenza.[45]
In the 21st century, a strain of H1N1 flu (since titled H1N1pdm09) which was antigenically very different from previous H1N1 strains, leading to a pandemic in 2009. Because of its close resemblance to some strains circulating in pigs, this became known as "Swine flu"[46]
Influenza A virus continues to circulate and evolve in birds and pigs. Almost all possible combinations of H (1 thru 16) and N (1 thru 11) have been isolated from wild birds.[26] As of June 2024, two particularly virulent IAV strains - H5N1 and H7N9 - are predominant in wild bird populations. These frequently cause outbreaks in domestic poultry, with occasional spillover infections in humans who are in close contact with poultry.[47][48]
Pandemic potential
[edit]Influenza viruses have a relatively high mutation rate that is characteristic of RNA viruses.[49] The segmentation of the influenza A virus genome facilitates genetic recombination by segment reassortment in hosts who become infected with two different strains of influenza viruses at the same time.[50][51] With reassortment between strains, an avian strain which does not affect humans may acquire characteristics from a different strain which enable it to infect and pass between humans - a zoonotic event.[52] It is thought that all influenza A viruses causing outbreaks or pandemics among humans since the 1900s originated from strains circulating in wild aquatic birds through reassortment with other influenza strains.[53][54] It is possible (though not certain) that pigs may act as an intermediate host for reassortment.[55]
Surveillance
[edit]The Global Influenza Surveillance and Response System (GISRS) is a global network of laboratories that monitor the spread of influenza with the aim to provide the World Health Organization with influenza control information and to inform vaccine development.[18] Several millions of specimens are tested by the GISRS network annually through a network of laboratories in 127 countries.[56] As well as human viruses, GISRS monitors avian, swine, and other potentially zoonotic influenza viruses.
Seasonal flu
[edit]
Flu season is an annually recurring time period characterized by the prevalence of an outbreak of influenza, caused either by Influenza A or by Influenza B. The season occurs during the cold half of the year in temperate regions; November through February in the northern hemisphere and May to October in the southern hemisphere. Flu seasons also exist in the tropics and subtropics, with variability from region to region.[58] Annually, about 3 to 5 million cases of severe illness and 290,000 to 650,000 deaths from seasonal flu occur worldwide.[2]
There are several possible reasons for the winter peak in temperate regions:
- During the winter, people spend more time indoors with the windows sealed, so they are more likely to breathe the same air as someone who has the flu and thus contract the virus.[59]
- Days are shorter during the winter, and lack of sunlight leads to low levels of vitamin D and melatonin, both of which require sunlight for their generation. This compromises our immune systems, which in turn decreases ability to fight the virus.[59]
- The influenza virus may survive better in colder, drier climates, and therefore be able to infect more people.[59]
- Cold air reduces the ability of the nasal membranes to resist infection.[60]
Zoonotic infections
[edit]A zoonosis a disease in a human caused by a pathogen (such as a bacterium, or virus) that has jumped from a non-human to a human.[61][62] Avian and pig influenza viruses can, on rare occasions, transmit to humans and cause zoonotic influenza virus infections; these infections are usually confined to people who have been in close contact with infected animals or material such as infected feces and meat, they do not spread to other humans. Symptoms of these infections in humans vary greatly; some are in asymptomatic or mild while others can cause severe disease, leading to severe pneumonia and death.[63] A wide range of Influenza A virus subtypes have been found to cause zoonotic disease.[63][64]
Zoonotic infections can be prevented by good hygiene, by preventing farmed animals from coming into contact with wild animals, and by using appropriate personal protective equipment.[62]
As of June 2024, there is concern about two subtypes of avian influenza which are circulating in wild bird populations worldwide, H5N1 and H7N9. Both of these have potential to devastate poultry stocks, and both have jumped to humans with relatively high case fatality rates.[64] H5N1 in particular has infected a wide range of mammals and may be adapting to mammalian hosts.[65]
Prevention and treatment
[edit]Vaccine
[edit]As of June 2024, the influenza viruses which circulate widely in humans are IAV subtypes H1N1 and H2N3, together with Influenza B.[66] Annual vaccination is the primary and most effective way to prevent influenza and influenza-associated complications, especially for high-risk groups.[67] Vaccines against the flu are trivalent or quadrivalent, providing protection against the dominant strains of IAV(H1N1) and IAV(H3N2), and one or two influenza B virus strains; the formulation is continually reviewed in order to match the predominant strains in circulation.[68][69]
Poultry and other animals - it is possible to vaccinate poultry and pigs against specific strains of influenza. Vaccination should be combined with other control measures such as infection monitoring, early detection and biosecurity.[70][71][72]
Treatment
[edit]The main treatment for mild influenza is supportive; rest, fluids, and over-the-counter medicines to alleviate symptoms while the body's own immune system works to recover from infection. Antiviral drugs are recommended for those with severe symptoms, or for those who are at risk of developing complications such as pneumonia.[73][2]
Signs and symptoms
[edit]Humans
[edit]
The symptoms of seasonal flu are similar to those of a cold, although usually more severe and less likely to include a runny nose.[77] The onset of symptoms is sudden, and initial symptoms are predominately non-specific: a sudden fever; muscle aches; cough; fatigue; sore throat; headache; difficulty sleeping; loss of appetite; diarrhoea or abdominal pain; nausea and vomiting.[78]
Humans can rarely become infected with strains of avian or swine influenza, usually as a result of close contact with infected animals or contaminated material; symptoms generally resemble seasonal flu but occasionally can be severe including death.[12][13]
Other animals
[edit]Birds
[edit]Some species of wild aquatic birds act as natural asymptomatic carriers of a large variety of influenza A viruses, which they can spread over large distances in their annual migration.[79] Symptoms of avian influenza vary according to both the strain of virus underlying the infection, and on the species of bird affected. Symptoms of influenza in birds may include swollen head, watery eyes, unresponsiveness, lack of coordination, respiratory distress such as sneezing or gurgling.[80]
Highly pathogenic avian influenza
[edit]Because of the impact of avian influenza on economically important chicken farms, avian virus strains are classified as either highly pathogenic (and therefore potentially requiring vigorous control measures) or low pathogenic. The test for this is based solely on the effect on chickens - a virus strain is highly pathogenic avian influenza (HPAI) if 75% or more of chickens die after being deliberately infected with it, or if it is genetically similar to such a strain. The alternative classification is low pathogenic avian influenza (LPAI).[81] Classification of a virus strain as either LPAI or HPAI is based on the severity of symptoms in domestic chickens and does not predict severity of symptoms in other species. Chickens infected with LPAI display mild symptoms or are asymptomatic, whereas HPAI causes serious breathing difficulties, significant drop in egg production, and sudden death.[82]
Since 2006, the World Organization for Animal Health requires all detections of LPAI H5 and H7 subtypes to be reported because of their potential to mutate into highly pathogenic strains.[83]
Pigs
[edit]Signs of swine flu in pigs can include fever, depression, coughing (barking), discharge from the nose or eyes, sneezing, breathing difficulties, eye redness or inflammation, and going off feed. Some pigs infected with influenza, however, may show no signs of illness at all. Swine flu subtypes are principally H1N1, H1N2, and H3N2;[84] it is spread either through close contact between animals or by the movement of contaminated equipment between farms.[85] Humans who are in close contact with pigs can sometimes become infected.[86]
Horses
[edit]Equine influenza can affect horses, donkeys, and mules;[87] it has a very high rate of transmission among horses, and a relatively short incubation time of one to three days.[88] Clinical signs of equine influenza include fever, nasal discharge, have a dry, hacking cough, depression, loss of appetite and weakness.[88] EI is caused by two subtypes of influenza A viruses: H7N7 and H3N8, which have evolved from avian influenza A viruses.[89]
Dogs
[edit]Most animals infected with canine influenza A will show symptoms such as coughing, runny nose, fever, lethargy, eye discharge, and a reduced appetite lasting anywhere from 2–3 weeks.[90] There are two different influenza A dog flu viruses: one is an H3N8 virus and the other is an H3N2 virus.[90] The H3N8 strain has evolved from an equine influenza avian virus which has adapted to sustained transmission among dogs. The H3N2 strain is derived from an avian influenza which jumped to dogs in 2004 in either Korea or China.[90] It is likely that the virus persists in both animal shelters and kennels, as well as in farms where dogs are raised for meat production.[91]
Bats
[edit]The first bat flu virus, IAV(H17N10), was first discovered in 2009 in little yellow-shouldered bats (Sturnira lilium) in Guatemala.[92] In 2012 a second bat influenza A virus IAV(H18N11) was discovered in flat-faced fruit-eating bats (Artibeus planirostris) from Peru.[93][94][95] Bat influenza viruses have been found to be poorly adapted to non-bat species.[96]
Forschung
[edit]Influenza research includes efforts to understand how influenza viruses enter hosts, the relationship between influenza viruses and bacteria, how influenza symptoms progress, and why some influenza viruses are deadlier than others.[97] Past pandemics, and especially the 1918 pandemic, are the subject of much research to understand and prevent flu pandemics.[98][99]
The World Health Organization has published a Research Agenda with five streams:[100]
- Stream 1. Reducing the risk of emergence of pandemic influenza. This stream is entirely focused on preventing and limiting pandemic influenza; this includes research into what characteristics make a strain either mild or deadly, worldwide surveillance of influenza A viruses with pandemic potential, and the prevention and management of potentially zoonotic influenza in domestic and farmed animals.[100]
- Stream 2. Limiting the spread of pandemic, zoonotic and seasonal epidemic influenza. This is more broadly targeted at both pandemic and seasonal influenza, looking at the transmission of the virus between people and the ways in which it can spread globally, as well as the environmental and social factors which affect transmission.[100]
- Stream 3. Minimizing the impact of pandemic, zoonotic, and seasonal epidemic influenza. This is principally concerned with vaccination - improving the effectiveness of vaccines, vaccine technology, as well as the speed with which an effective vaccine can be developed and ways in which vaccines can be manufactured and delivered worldwide.[100]
- Stream 4. Optimizing the treatment of patients. This stream aims to reduce the impact of influenza by looking at methods of treatment, vulnerable groups, genetic predispositions, the interaction of influenza infection with other diseases, and influenza sequelae.[100]
- Stream 5. Promoting the development and application of modern public health tools.[100] Aiming to improve the ways in which public policy can combat influenza; this includes the introduction of new technologies, epidemic and pandemic modelling, and the communication of accurate and trustworthy information to the public.[100]
See also
[edit]References
[edit]- ^ "Taxonomy". International Committee on Taxonomy of Viruses (ICTV). Archived from the original on 20 March 2020. Retrieved 19 July 2018.
- ^ a b c d "Influenza (Seasonal)". World Health Organization. Retrieved 22 June 2024.
- ^ Runstadler JA, Puryear W (2020). "A Brief Introduction to Influenza A Virus in Marine Mammals". Animal Influenza Virus. Methods in Molecular Biology (Clifton, N.J.). Vol. 2123. pp. 429–450. doi:10.1007/978-1-0716-0346-8_33. ISBN 978-1-0716-0345-1. ISSN 1940-6029. PMID 32170708.
- ^ a b "Influenza A Subtypes and the Species Affected | Seasonal Influenza (Flu) | CDC". Centers for Disease Control and Prevention. 13 May 2024. Retrieved 17 June 2024.
- ^ Shao W, Li X, Goraya MU, Wang S, Chen JL (August 2017). "Evolution of Influenza A Virus by Mutation and Re-Assortment". International Journal of Molecular Sciences. 18 (8): 1650. doi:10.3390/ijms18081650. PMC 5578040. PMID 28783091.
- ^ Eisfeld AJ, Neumann G, Kawaoka Y (January 2015). "At the centre: influenza A virus ribonucleoproteins". Nature Reviews. Microbiology. 13 (1): 28–41. doi:10.1038/nrmicro3367. PMC 5619696. PMID 25417656.
- ^ CDC (1 February 2024). "Influenza Type A Viruses". Centers for Disease Control and Prevention. Retrieved 3 May 2024.
- ^ "FluGlobalNet - Avian Influenza". science.vla.gov.uk. Retrieved 5 June 2024.
- ^ CDC (30 March 2023). "Types of Influenza Viruses". Centers for Disease Control and Prevention. Retrieved 17 June 2024.
- ^ CDC (11 June 2024). "Avian Influenza Type A Viruses". Avian Influenza (Bird Flu). Retrieved 17 June 2024.
- ^ "Flu". National Health Service. 23 October 2017. Retrieved 17 June 2024.
- ^ a b "Avian influenza: guidance, data and analysis". GOV.UK. 18 November 2021. Retrieved 9 May 2024.
- ^ a b "Swine influenza in humans". European Centre for Disease Prevention and Control (ECDC). 20 September 2017. Retrieved 17 June 2024.
- ^ Joseph U, Su YC, Vijaykrishna D, Smith GJ (January 2017). "The ecology and adaptive evolution of influenza A interspecies transmission". Influenza and Other Respiratory Viruses. 11 (1): 74–84. doi:10.1111/irv.12412. PMC 5155642. PMID 27426214.
- ^ "Avian influenza (bird flu)". European Medicines Agency. 12 June 2024. Retrieved 18 June 2024.
- ^ "Avian influenza (bird flu) vaccination". UK Government - Department for Environment Food & Rural Affairs. 5 June 2023. Retrieved 18 June 2024.
- ^ CDC (20 March 2024). "What You Should Know about Flu Antiviral Drugs". Centers for Disease Control and Prevention. Retrieved 18 June 2024.
- ^ a b Lee K, Fang J (2013). Historical Dictionary of the World Health Organization. Rowman & Littlefield. ISBN 9780810878587.
- ^ "70 years of GISRS – the Global Influenza Surveillance & Response System". World Health Organization. 19 September 2022. Retrieved 13 June 2024.
- ^ Masurel N (1969). "Serological characteristics of a "new" serotype of influenza A virus: the Hong Kong strain". Bulletin of the World Health Organization. 41 (3): 461–468. PMC 2427714. PMID 5309456.
- ^ Johnson J, Higgins A, Navarro A, Huang Y, Esper FL, Barton N, et al. (February 2012). "Subtyping influenza A virus with monoclonal antibodies and an indirect immunofluorescence assay". Journal of Clinical Microbiology. 50 (2): 396–400. doi:10.1128/JCM.01237-11. PMC 3264186. PMID 22075584.
- ^ a b "Influenza Type A Viruses and Subtypes". Centers for Disease Control and Prevention. 2 April 2013. Archived from the original on 1 June 2021. Retrieved 13 June 2013.
- ^ Tong S, Zhu X, Li Y, Shi M, Zhang J, Bourgeois M, et al. (October 2013). "New world bats harbor diverse influenza A viruses". PLOS Pathogens. 9 (10): e1003657. doi:10.1371/journal.ppat.1003657. PMC 3794996. PMID 24130481.
- ^ "Influenza Virus Genome Sequencing and Genetic Characterization | CDC". Centers for Disease Prevention and Control. 27 February 2024. Retrieved 19 June 2024.
- ^ Eisfeld AJ, Neumann G, Kawaoka Y (January 2015). "At the centre: influenza A virus ribonucleoproteins". Nature Reviews. Microbiology. 13 (1): 28–41. doi:10.1038/nrmicro3367. PMC 5619696. PMID 25417656.
- ^ a b "FluGlobalNet - Avian Influenza". science.vla.gov.uk. Retrieved 5 June 2024.
- ^ "Influenza A Subtypes and the Species Affected | Seasonal Influenza (Flu) | CDC". Centers for Disease Control and Prevention. 17 June 2024. Retrieved 18 June 2024.
- ^ "A revision of the system of nomenclature for influenza viruses: a WHO memorandum". Bulletin of the World Health Organization. 58 (4): 585–591. 1980. PMC 2395936. PMID 6969132.
This Memorandum was drafted by the signatories listed on page 590 on the occasion of a meeting held in Geneva in February 1980.
- ^ a b c d e "Technical note: Influenza virus nomenclature". Pan American Health Organization. 11 January 2023. Archived from the original on 10 August 2023. Retrieved 27 May 2024.
- ^ Dadonaite B, Vijayakrishnan S, Fodor E, Bhella D, Hutchinson EC (August 2016). "Filamentous influenza viruses". The Journal of General Virology. 97 (8): 1755–1764. doi:10.1099/jgv.0.000535. PMC 5935222. PMID 27365089.
- ^ a b c d Bouvier NM, Palese P (September 2008). "The biology of influenza viruses". Vaccine. 26 (Suppl 4): D49–D53. doi:10.1016/j.vaccine.2008.07.039. PMC 3074182. PMID 19230160.
- ^ a b c Shaffer C (7 March 2018). "Influenza A Structure". News-Medical. Retrieved 18 June 2024.
- ^ a b c "Virology of human influenza". World Health Organization. 13 May 2010. Retrieved 19 June 2024.
- ^ a b c Krammer F, Smith GJ, Fouchier RA, Peiris M, Kedzierska K, Doherty PC, et al. (June 2018). "Influenza". Nature Reviews. Disease Primers. 4 (1): 3. doi:10.1038/s41572-018-0002-y. PMC 7097467. PMID 29955068.
- ^ a b c Jakob C, Paul-Stansilaus R, Schwemmle M, Marquet R, Bolte H (September 2022). "The influenza A virus genome packaging network - complex, flexible and yet unsolved". Nucleic Acids Research. 50 (16): 9023–9038. doi:10.1093/nar/gkac688. PMC 9458418. PMID 35993811.
- ^ a b c Dou D, Revol R, Östbye H, Wang H, Daniels R (20 July 2018). "Influenza A Virus Cell Entry, Replication, Virion Assembly and Movement". Frontiers in Immunology. 9: 1581. doi:10.3389/fimmu.2018.01581. PMC 6062596. PMID 30079062.
- ^ a b c Rashid F, Xie Z, Li M, Xie Z, Luo S, Xie L (13 December 2023). "Roles and functions of IAV proteins in host immune evasion". Frontiers in Immunology. 14: 1323560. doi:10.3389/fimmu.2023.1323560. PMC 10751371. PMID 38152399.
- ^ Decroly E, Canard B (June 2017). "Biochemical principles and inhibitors to interfere with viral capping pathways". Current Opinion in Virology. 24: 87–96. doi:10.1016/j.coviro.2017.04.003. PMC 7185569. PMID 28527860.
- ^ Rampersad S, Tennant P (2018). "Replication and Expression Strategies of Viruses". Viruses: 55–82. doi:10.1016/B978-0-12-811257-1.00003-6. PMC 7158166.
- ^ Mahmoud SM, Alison M, Knobler SL, eds. (2005). "1, The Story of Influenza.". The Threat of Pandemic Influenza: Are We Ready? Workshop Summary. Institute of Medicine (US) Forum on Microbial Threats. Washington (DC): National Academies Press (US).
- ^ Suzuki Y, Nei M (April 2002). "Origin and evolution of influenza virus hemagglutinin genes". Molecular Biology and Evolution. 19 (4). Ocford Academic: 501–509. doi:10.1093/oxfordjournals.molbev.a004105. PMID 11919291.
- ^ a b "The History of Influenza". www.flu.com. Retrieved 20 June 2024.
- ^ Shimizu K (October 1997). "[History of influenza epidemics and discovery of influenza virus]". Nihon Rinsho. Japanese Journal of Clinical Medicine. 55 (10): 2505–2511. PMID 9360364.
- ^ "CDC Archives : 1918 Pandemic (H1N1 virus)". Centers for Disease Control and Prevention. 20 March 2019. Retrieved 20 June 2024.
- ^ a b Knobler SL, Mack A, Mahmoud A, Lemon SM, et al. (Institute of Medicine (US) Forum on Microbial Threats) (2005). "The Story of Influenza". The Threat of Pandemic Influenza: Are We Ready? Workshop Summary. National Academies Press (US). Retrieved 20 June 2024.
- ^ "2009 H1N1 Pandemic (H1N1pdm09 virus)". CDC Archive: Centers for Disease Control and Prevention. 11 June 2019. Retrieved 21 June 2024.
- ^ "The next pandemic: H5N1 and H7N9 influenza?". Gavi, the Vaccine Alliance. 26 March 2021. Retrieved 21 June 2024.
- ^ "Influenza (Avian and other zoonotic)". World Health Organization. 3 October 2023. Retrieved 21 June 2024.
- ^ Sanjuán R, Nebot MR, Chirico N, Mansky LM, Belshaw R (October 2010). "Viral mutation rates". Journal of Virology. 84 (19): 9733–9748. doi:10.1128/JVI.00694-10. PMC 2937809. PMID 20660197.
- ^ Kou Z, Lei FM, Yu J, Fan ZJ, Yin ZH, Jia CX, et al. (December 2005). "New genotype of avian influenza H5N1 viruses isolated from tree sparrows in China". Journal of Virology. 79 (24): 15460–15466. doi:10.1128/JVI.79.24.15460-15466.2005. PMC 1316012. PMID 16306617.
- ^ The World Health Organization Global Influenza Program Surveillance Network (October 2005). "Evolution of H5N1 avian influenza viruses in Asia". Emerging Infectious Diseases. 11 (10): 1515–1521. doi:10.3201/eid1110.050644. PMC 3366754. PMID 16318689. Figure 1 shows a diagramatic representation of the genetic relatedness of Asian H5N1 hemagglutinin genes from various isolates of the virus
- ^ CDC (15 May 2024). "Transmission of Bird Flu Viruses Between Animals and People". Centers for Disease Control and Prevention. Retrieved 10 June 2024.
- ^ Taubenberger JK, Morens DM (April 2010). "Influenza: the once and future pandemic". Public Health Reports. 125 (Suppl 3): 16–26. doi:10.1177/00333549101250S305. PMC 2862331. PMID 20568566.
- ^ Webster RG, Bean WJ, Gorman OT, Chambers TM, Kawaoka Y (March 1992). "Evolution and ecology of influenza A viruses". Microbiological Reviews. 56 (1): 152–179. doi:10.1128/mr.56.1.152-179.1992. PMC 372859. PMID 1579108.
- ^ "Factsheet on swine influenza in humans and pigs". European Centre for Disease Control. 15 June 2017. Retrieved 13 June 2024.
- ^ "70 years of GISRS – the Global Influenza Surveillance & Response System". World Health Organization. 19 September 2022. Retrieved 13 June 2024.
- ^ CDC U.S. influenza season summary with weekly updates See section 'Pneumonia and Influenza (P&I) Mortality Surveillance' www.cdc.gov, accessed 30 September 2020
- ^ Hirve S, Newman LP, Paget J, Azziz-Baumgartner E, Fitzner J, Bhat N, et al. (27 April 2016). "Influenza Seasonality in the Tropics and Subtropics - When to Vaccinate?". PLOS ONE. 11 (4): e0153003. Bibcode:2016PLoSO..1153003H. doi:10.1371/journal.pone.0153003. PMC 4847850. PMID 27119988.
- ^ a b c "The Reason for the Season: why flu strikes in winter". Science in the News, a Graduate Student Group at the Harvard Graduate School of the Arts and Sciences. 1 December 2014. Retrieved 21 June 2024.
- ^ LaMotte S (6 December 2022). "Scientists finally know why people get more colds and flu in winter". CNN. Retrieved 21 June 2024.
- ^ "zoonosis". Merriam-Webster.com Dictionary. Merriam-Webster. Retrieved 29 March 2019.
- ^ a b "Zoonoses - Key Facts". World Health Organization. 29 July 2020. Retrieved 24 June 2024.
- ^ a b "Zoonotic influenza - Annual Epidemiological Report for 2022". www.ecdc.europa.eu. 23 May 2023. Retrieved 24 June 2024.
- ^ a b "Global AIV with Zoonotic Potential". The Food and Agriculture Organization (FAO) of the United Nations. 29 July 2020. Retrieved 24 June 2024.
- ^ Plaza PI, Gamarra-Toledo V, Euguí JR, Lambertucci SA (March 2024). "Recent Changes in Patterns of Mammal Infection with Highly Pathogenic Avian Influenza A(H5N1) Virus Worldwide". Emerging Infectious Diseases. 30 (3): 444–452. doi:10.3201/eid3003.231098. PMC 10902543. PMID 38407173.
- ^ CDC (30 March 2023). "Types of Influenza Viruses". Centers for Disease Control and Prevention. Retrieved 22 June 2024.
- ^ Chow EJ, Doyle JD, Uyeki TM (June 2019). "Influenza virus-related critical illness: prevention, diagnosis, treatment". Critical Care. 23 (1): 214. doi:10.1186/s13054-019-2491-9. PMC 6563376. PMID 31189475.
- ^ Dharmapalan D (October 2020). "Influenza". Indian Journal of Pediatrics. 87 (10): 828–832. doi:10.1007/s12098-020-03214-1. PMC 7091034. PMID 32048225.
- ^ Sautto GA, Kirchenbaum GA, Ross TM (January 2018). "Towards a universal influenza vaccine: different approaches for one goal". Virology Journal. 15 (1): 17. doi:10.1186/s12985-017-0918-y. PMC 5785881. PMID 29370862.
- ^ "Vaccination of poultry against highly pathogenic avian influenza – Available vaccines and vaccination strategies". efsa.europa.eu. 10 October 2023. Retrieved 9 May 2024.
- ^ "Making a Candidate Vaccine Virus (CVV) for a HPAI (Bird Flu) Virus". Centers for Disease Control. 3 June 2024. Retrieved 15 June 2024.
- ^ "What People Who Raise Pigs Need To Know About Influenza (Flu) | CDC". Centers for Disease Control and Prevention. 19 October 2023. Retrieved 22 June 2024.
- ^ CDC (22 March 2024). "Take everyday precautions to protect others while sick". Centers for Disease Control and Prevention. Retrieved 22 June 2024.
- ^ "Flu Symptoms & Diagnosis". U.S. Centers for Disease Control and Prevention (CDC). 10 July 2019. Archived from the original on 27 December 2019. Retrieved 24 January 2020.
- ^ "Flu Symptoms & Complications". U.S. Centers for Disease Control and Prevention (CDC). 26 February 2019. Archived from the original on 1 August 2020. Retrieved 6 July 2019.
- ^ Call SA, Vollenweider MA, Hornung CA, Simel DL, McKinney WP (February 2005). "Does this patient have influenza?". JAMA. 293 (8): 987–997. doi:10.1001/jama.293.8.987. PMID 15728170.
- ^ CDC (29 September 2022). "Cold Versus Flu". Centers for Disease Control and Prevention. Retrieved 25 June 2024.
- ^ "Flu". National Health Service UK. 9 August 2023. Retrieved 25 June 2024.
- ^ "Bird flu (avian influenza): how to spot and report it in poultry or other captive birds". Department for Environment, Food & Rural Affairs and Animal and Plant Health Agency. 13 December 2022. Retrieved 6 May 2024.
- ^ "Avian flu". The Royal Society for the Protection of Birds (RSPB). Retrieved 25 June 2024.
- ^ Alexander DJ, Brown IH (April 2009). "History of highly pathogenic avian influenza". Revue Scientifique et Technique. 28 (1): 19–38. doi:10.20506/rst.28.1.1856. PMID 19618616.
- ^ CDC (14 June 2022). "Avian Influenza in Birds". Centers for Disease Control and Prevention. Retrieved 6 May 2024.
- ^ "National H5/H7 Avian Influenza surveillance plan". United States Department of Agriculture. Animal Plant Health Inspection Service. October 2013.
- ^ "Factsheet on swine influenza in humans and pigs". European Centre for Disease Prevention and Control. 15 June 2017. Retrieved 25 June 2024.
- ^ "Key Facts about Swine Influenza (Swine Flu) in Pigs | CDC". Centers for Disease Control and Prevention. 3 October 2018. Retrieved 25 June 2024.
- ^ "2023: outbreaks of swine influenza". World Health Organization. 30 March 2024. Retrieved 25 June 2024.
- ^ "Equine influenza". WOAH - World Organisation for Animal Health. Retrieved 25 June 2024.
- ^ a b "Equine Influenza: Respiratory Diseases of Horses: Merck Veterinary Manual". www.merckvetmanual.com. Archived from the original on 15 November 2016. Retrieved 4 December 2016.
- ^ CDC (5 May 2023). "Horse Flu". Centers for Disease Control and Prevention. Retrieved 25 June 2024.
- ^ a b c "Key Facts about Canine Influenza (Dog Flu) | Seasonal Influenza (Flu) | CDC". Centers for Disease Protection and Control. 29 August 2023. Retrieved 25 June 2024.
- ^ Wasik BR, Voorhees IE, Parrish CR (January 2021). "Canine and Feline Influenza". Cold Spring Harbor Perspectives in Medicine. 11 (1): a038562. doi:10.1101/cshperspect.a038562. PMC 7778219. PMID 31871238.
- ^ "Bat Influenza (Flu)". cdc.gov. Retrieved 30 June 2020.
- ^ "Characterization of bat influenza viruses". uniklinik-freiburg.de. Retrieved 30 June 2020.
- ^ "New flu virus found in bats". Nature. 503 (7475): 169. 2013. doi:10.1038/503169e. Retrieved 30 June 2020.
- ^ Ciminski K, Pfaff F, Beer M, Schwemmle M (April 2020). "Bats reveal the true power of influenza A virus adaptability". PLOS Pathogens. 16 (4): e1008384. doi:10.1371/journal.ppat.1008384. PMC 7161946. PMID 32298389.
- ^ Ciminski K, Ran W, Gorka M, Lee J, Malmlov A, Schinköthe J, et al. (December 2019). "Bat influenza viruses transmit among bats but are poorly adapted to non-bat species". Nature Microbiology. 4 (12): 2298–2309. doi:10.1038/s41564-019-0556-9. PMC 7758811. PMID 31527796. S2CID 202580293.
- ^ "Influenza Basic Research". National Institute of Allergy and Infectious Diseases. 13 March 2017. Archived from the original on 10 June 2024. Retrieved 24 March 2021.
- ^ Potter CW (October 2001). "A history of influenza". Journal of Applied Microbiology. 91 (4): 572–579. doi:10.1046/j.1365-2672.2001.01492.x. PMID 11576290. S2CID 26392163.
- ^ Taubenberger JK, Baltimore D, Doherty PC, Markel H, Morens DM, Webster RG, et al. (November 2012). "Reconstruction of the 1918 influenza virus: unexpected rewards from the past". mBio. 3 (5). doi:10.1128/mBio.00201-12. PMC 3448162. PMID 22967978.
- ^ a b c d e f g "WHO public health research agenda for influenza: 2017 update". World Health Organization. Geneva. 2017. Retrieved 28 June 2024.
Further reading
[edit]- Official sources
- Information on Bird Flu US Centers for Disease Control and Prevention (CDC)
- Pandemic Influenza US CDC
- General information
- Web focus: Warnings of a Flu Pandemic Nature
- Nature Reports: Homepage: Avian Flu
- Beigel JH, Farrar J, Han AM, Hayden FG, Hyer R, de Jong MD, et al. (September 2005). "Avian influenza A (H5N1) infection in humans". The New England Journal of Medicine. 353 (13): 1374–1385. CiteSeerX 10.1.1.730.7890. doi:10.1056/NEJMra052211. PMID 16192482.
- Pandemic Influenza: Domestic Preparedness Efforts Congressional Research Service Report on Pandemic Preparedness.
- Mahmoud (2005). Stacey L. Knobler, Alison Mack, Mahmoud A, Stanley M. Lemon (eds.). The threat of pandemic influenza : are we ready? : workshop summary / prepared for Forum on Microbial Threats, Board on Global Health. The National Academies Press. p. 285. ISBN 0-309-09504-2.
Highly pathogenic avian influenza virus is on every top ten list available for potential agricultural bioweapon agents
- Mahmoud AA, Institute of Medicine, Knobler S, Mack A (2005). The Threat of Pandemic Influenza: Are We Ready?: Workshop Summary. Washington, D.C.: National Academies Press. ISBN 978-0-309-09504-4.
- Links to Bird Flu pictures (Hardin MD/Univ of Iowa)
- Kawaoka Y (2006). Influenza Virology: Current Topics. Caister Academic Pr. ISBN 978-1-904455-06-6.
- Sobrino F, Mettenleiter T (2008). Animal Viruses: Molecular Biology. Caister Academic Press. ISBN 978-1-904455-22-6.
