Colesevelam: Difference between revisions
update infobox, links, portal |
Rescuing 4 sources and tagging 0 as dead.) #IABot (v2.0.9.5 |
||
| (17 intermediate revisions by 11 users not shown) | |||
| Line 1: | Line 1: | ||
{{Short description|Pharmaceutical drug}} |
|||
{{Use dmy dates|date=August 2024}} |
|||
{{cs1 config |name-list-style=vanc |display-authors=6}} |
|||
{{Drugbox |
{{Drugbox |
||
| Verifiedfields = changed |
| Verifiedfields = changed |
||
| Line 9: | Line 12: | ||
| Drugs.com = {{drugs.com|monograph|colesevelam-hydrochloride}} |
| Drugs.com = {{drugs.com|monograph|colesevelam-hydrochloride}} |
||
| MedlinePlus = a699050 |
| MedlinePlus = a699050 |
||
| licence_EU = yes |
|||
| DailyMedID = Colesevelam |
| DailyMedID = Colesevelam |
||
| pregnancy_AU = <!-- A / B1 / B2 / B3 / C / D / X --> |
| pregnancy_AU = <!-- A / B1 / B2 / B3 / C / D / X --> |
||
| ⚫ | |||
| pregnancy_US = N |
|||
| ⚫ | |||
| routes_of_administration = [[Oral administration|By mouth]] |
| routes_of_administration = [[Oral administration|By mouth]] |
||
| ATC_prefix = C10 |
| ATC_prefix = C10 |
||
| Line 22: | Line 23: | ||
| legal_UK = <!-- GSL / P / POM / CD / Class A, B, C --> |
| legal_UK = <!-- GSL / P / POM / CD / Class A, B, C --> |
||
| legal_US = Rx-only |
| legal_US = Rx-only |
||
| legal_US_comment = <ref>{{cite web | title=Welchol- colesevelam hydrochloride tablet, film coated; Welchol- colesevelam hydrochloride for suspension | website=DailyMed | date=7 September 2022 | url=https://dailymed.nlm.nih.gov/dailymed/drugInfo.cfm?setid=89cbd0a3-2d1b-41db-8c97-35ec67b7b09f | access-date=16 August 2024 | archive-date=3 February 2023 | archive-url=https://web.archive.org/web/20230203105429/https://dailymed.nlm.nih.gov/dailymed/drugInfo.cfm?setid=89cbd0a3-2d1b-41db-8c97-35ec67b7b09f | url-status=live }}</ref><ref>{{cite web | title=Welchol- colesevelam hydrochloride tablet, film coated; Welchol- colesevelam hydrochloride for suspension; Welchol- colesevelam hydrochloride bar, chewable | website=DailyMed | date=10 June 2022 | url=https://dailymed.nlm.nih.gov/dailymed/drugInfo.cfm?setid=4a06d3b2-7229-4398-baba-5d0a72f63821 | access-date=16 August 2024 | archive-date=20 May 2024 | archive-url=https://web.archive.org/web/20240520083357/https://dailymed.nlm.nih.gov/dailymed/drugInfo.cfm?setid=4a06d3b2-7229-4398-baba-5d0a72f63821 | url-status=live }}</ref> |
|||
| legal_EU = Rx-only |
| legal_EU = Rx-only |
||
| legal_EU_comment = <ref>{{cite web | title=Cholestagel EPAR | website=European Medicines Agency (EMA) | date=10 March 2004 | url=https://www.ema.europa.eu/en/medicines/human/EPAR/cholestagel | access-date=16 August 2024 | archive-date=15 April 2021 | archive-url=https://web.archive.org/web/20210415004141/https://www.ema.europa.eu/en/medicines/human/EPAR/cholestagel | url-status=live }}</ref> |
|||
| legal_status = Rx-only |
| legal_status = Rx-only |
||
<!--Pharmacokinetic data --> |
<!--Pharmacokinetic data --> |
||
| bioavailability = N/A |
| bioavailability = N/A |
||
| protein_bound = |
| protein_bound = |
||
| metabolism = Colesevelam is not absorbed and not |
| metabolism = Colesevelam is not absorbed and not metabolized |
||
| elimination_half-life = N/A (non-systemic drug) |
| elimination_half-life = N/A (non-systemic drug) |
||
| excretion = By intestines only, colesevelam is non-systemic |
| excretion = By intestines only, colesevelam is non-systemic |
||
<!--Identifiers --> |
<!--Identifiers --> |
||
| Line 55: | Line 59: | ||
}} |
}} |
||
'''Colesevelam''' is a [[bile acid sequestrant]] administered orally. It was developed by GelTex Pharmaceuticals and later acquired by Genzyme. |
'''Colesevelam''' is a [[bile acid sequestrant]] administered orally. It was developed by GelTex Pharmaceuticals and later acquired by Genzyme. It is marketed in the US by [[Daiichi Sankyo]] under the brand name '''Welchol''' and elsewhere by Genzyme as '''Cholestagel'''. In Canada, it is marketed by [[Valeant]] as '''Lodalis'''. |
||
==Clinical use== |
==Clinical use== |
||
Colesevelam is indicated as an adjunct to diet and exercise to reduce elevated [[low-density lipoprotein]] cholesterol (LDL-C) in patients with primary [[hyperlipidemia]] as monotherapy and to improve glycemic control in adults with type 2 [[diabetes mellitus]],<ref>{{ |
Colesevelam is indicated as an adjunct to diet and exercise to reduce elevated [[low-density lipoprotein]] cholesterol (LDL-C) in patients with primary [[hyperlipidemia]] as monotherapy and to improve glycemic control in adults with type 2 [[diabetes mellitus]],<ref>{{cite journal | vauthors = Fonseca VA, Rosenstock J, Wang AC, Truitt KE, Jones MR | title = Colesevelam HCl improves glycemic control and reduces LDL cholesterol in patients with inadequately controlled type 2 diabetes on sulfonylurea-based therapy | journal = Diabetes Care | volume = 31 | issue = 8 | pages = 1479–1484 | date = August 2008 | pmid = 18458145 | pmc = 2494667 | doi = 10.2337/dc08-0283 }}</ref> including in combination with a [[statin]]. The expanded use of colesevelam in adults with type 2 diabetes mellitus is an example of [[drug repositioning]].{{cn|date=March 2022}} |
||
Colesevelam is one of the [[Bile acid sequestrant|bile-acid sequestrants]], which along with [[niacin]] and the statins, are the three main types of cholesterol-lowering agents. The statins are considered the first-line agents. This is because of the larger body of evidence supporting statins' ability to prevent cardiovascular disease, as well as the prominent side effects from the other two types, including bloating and constipation (bile-acid sequestrants) and skin flushing (niacin). These side effects often lead to low patient compliance.<ref>Principles and Practice of Endocrinology and Metabolism, 2000, ed. Becker, chapter 163</ref> |
Colesevelam is one of the [[Bile acid sequestrant|bile-acid sequestrants]], which along with [[Niacin (substance)|niacin]] and the statins, are the three main types of cholesterol-lowering agents. The statins are considered the first-line agents. This is because of the larger body of evidence supporting statins' ability to prevent cardiovascular disease, as well as the prominent side effects from the other two types, including bloating and constipation (bile-acid sequestrants) and skin flushing (niacin). These side effects often lead to low patient compliance.<ref>Principles and Practice of Endocrinology and Metabolism, 2000, ed. Becker, chapter 163</ref> |
||
Colesevelam can be used instead of [[cholestyramine]] in symptomatic chronic [[diarrhea]] due to [[bile salt malabsorption]] ([[bile acid diarrhea]]), which can be a primary condition, or secondary to [[Crohn's disease]] or the [[postcholecystectomy syndrome]].<ref>{{cite journal| |
Colesevelam can be used instead of [[cholestyramine]] in symptomatic chronic [[diarrhea]] due to [[bile salt malabsorption]] ([[bile acid diarrhea]]), which can be a primary condition, or secondary to [[Crohn's disease]] or the [[postcholecystectomy syndrome]].<ref>{{cite journal | vauthors = Puleston J, Morgan H, Andreyev J | title = New treatment for bile salt malabsorption | journal = Gut | volume = 54 | issue = 3 | pages = 441–442 | date = March 2005 | pmid = 15711000 | pmc = 1774391 | doi = 10.1136/gut.2004.054486 }}</ref><ref>{{cite journal | vauthors = Wedlake L, Thomas K, Lalji A, Anagnostopoulos C, Andreyev HJ | title = Effectiveness and tolerability of colesevelam hydrochloride for bile-acid malabsorption in patients with cancer: a retrospective chart review and patient questionnaire | journal = Clinical Therapeutics | volume = 31 | issue = 11 | pages = 2549–2558 | date = November 2009 | pmid = 20109999 | doi = 10.1016/j.clinthera.2009.11.027 }}</ref><ref name="pmid24953836">{{cite journal | vauthors = Beigel F, Teich N, Howaldt S, Lammert F, Maul J, Breiteneicher S, Rust C, Göke B, Brand S, Ochsenkühn T | title = Colesevelam for the treatment of bile acid malabsorption-associated diarrhea in patients with Crohn's disease: a randomized, double-blind, placebo-controlled study | journal = Journal of Crohn's & Colitis | volume = 8 | issue = 11 | pages = 1471–1479 | date = November 2014 | pmid = 24953836 | doi = 10.1016/j.crohns.2014.05.009 | doi-access = free }}</ref> |
||
==Constituents== |
==Constituents== |
||
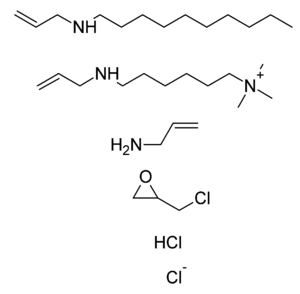
Colesevelam is a modified polyallylamine. It is made by crosslinking polyallylamine with epichlorohydrin, and then modifying it with bromodecane and (6-bromohexyl)trimethylammonium bromide. The bromide ions are then replaced with chloride ions when the material is washed.<ref>US Patent 5,607,669</ref> |
Colesevelam is a modified polyallylamine. It is made by crosslinking polyallylamine with epichlorohydrin, and then modifying it with {{chem name|bromodecane}} and (6-bromohexyl)trimethylammonium bromide. The bromide ions are then replaced with chloride ions when the material is washed.<ref>US Patent 5,607,669</ref> |
||
The constituents of the polymer colesevelam shown as subunits that do not exist per se in the final product are: |
The constituents of the polymer colesevelam shown as subunits that do not exist per se in the final product are: |
||
| Line 74: | Line 78: | ||
==Mechanism of action== |
==Mechanism of action== |
||
Colesevelam is part of a class of drugs known as bile acid sequestrants. Colesevelam hydrochloride, the active pharmaceutical ingredient in '''Welchol''', is a non-absorbed, lipid-lowering polymer that binds bile acids in the intestine, impeding their reabsorption. As the bile acid pool becomes depleted, the hepatic enzyme, cholesterol 7-α-hydroxylase, is upregulated, which increases the conversion of cholesterol to bile acids. This causes an increased demand for cholesterol in the liver cells, resulting in the dual effect of increasing transcription and activity of the cholesterol biosynthetic enzyme, HMG-CoA reductase, and increasing the number of hepatic LDL receptors. These compensatory effects result in increased clearance of LDL-C from the blood, resulting in decreased serum LDL-C levels. Serum TG levels may increase or remain unchanged.<ref>RxList |
Colesevelam is part of a class of drugs known as bile acid sequestrants. Colesevelam hydrochloride, the active pharmaceutical ingredient in '''Welchol''', is a non-absorbed, lipid-lowering polymer that binds bile acids in the intestine, impeding their reabsorption. As the bile acid pool becomes depleted, the hepatic enzyme, cholesterol 7-α-hydroxylase, is upregulated, which increases the conversion of cholesterol to bile acids. This causes an increased demand for cholesterol in the liver cells, resulting in the dual effect of increasing transcription and activity of the cholesterol biosynthetic enzyme, HMG-CoA reductase, and increasing the number of hepatic LDL receptors. These compensatory effects result in increased clearance of LDL-C from the blood, resulting in decreased serum LDL-C levels. Serum TG levels may increase or remain unchanged.<ref>{{cite web | work = RxList | url = http://www.rxlist.com/welchol-drug.htm | title = Welchol | access-date = 11 April 2009 | archive-date = 16 January 2018 | archive-url = https://web.archive.org/web/20180116171244/https://www.rxlist.com/welchol-drug.htm | url-status = live }}</ref> |
||
It is not yet known how Colesevelam works to help control blood sugar in people with type 2 diabetes. However, it is clear that the drug works within the digestive tract, since it is not absorbed into the rest of the body. |
|||
===Cholesterol=== |
|||
Since Colesevelam can lower total and LDL cholesterol levels (along with raising HDL - "good" cholesterol), taking it may decrease one's risk of developing certain health problems in the future. |
|||
Previous clinical research studies indicate individuals taking 3,800 mg to 4,500 mg of Colesevelam daily were able to: |
|||
* Reduce LDL cholesterol by 15 to 18 percent. |
|||
* Reduce total cholesterol by 7 to 10 percent. |
|||
* Raise HDL cholesterol by 3 percent. |
|||
The combination of Colesevelam with a HMG-CoA reductase inhibitor (known more commonly as a statin) can further lower cholesterol levels.<ref>eMedTV: [http://cholesterol.emedtv.com/welchol/welchol.html WelChol]</ref> |
|||
== Side effects == |
== Side effects == |
||
In controlled clinical studies involving approximately 1,400 patients, the following adverse reactions have been reported in patients treated with colesevelam. When reporting to the very common (≥ 1 / 10), common (≥ 1 / 100, 51/10), uncommon (≥ 1 / 1000, 51/100), rare (≥ 1/10.000, 51/1000) and distinction very rarely (51/10.000), including individual cases: |
In controlled clinical studies involving approximately 1,400 patients, the following adverse reactions have been reported in patients treated with colesevelam. When reporting to the very common (≥ 1 / 10), common (≥ 1 / 100, 51/10), uncommon (≥ 1 / 1000, 51/100), rare (≥ 1/10.000, 51/1000) and distinction very rarely (51/10.000), including individual cases:{{cn|date=March 2022}} |
||
*Investigations Common: serum triglyceride increased; Uncommon: serum transaminase increases |
* Investigations Common: serum triglyceride increased; Uncommon: serum transaminase increases |
||
*Nervous system disorders Common: headache |
* Nervous system disorders Common: headache |
||
*Gastrointestinal disorders Very Common: flatulence, constipation; Common: vomiting, diarrhea, dyspepsia, abdominal pain, stool abnormalities, nausea |
* Gastrointestinal disorders Very Common: flatulence, constipation; Common: vomiting, diarrhea, dyspepsia, abdominal pain, stool abnormalities, nausea |
||
*Musculoskeletal and connective tissue disorders Uncommon: myalgia |
* Musculoskeletal and connective tissue disorders Uncommon: myalgia |
||
The background incidence of flatulence and diarrhea was the same in patients in controlled clinical trials, and higher in the placebo group. Only constipation and dyspepsia were shown to occur in a higher percentage of patients who received Cholestagel, compared to the placebo group. Side effects were generally mild or moderate in severity. In the application of colesevelam in combination with statins, no unexpected frequent side effects occurred.<ref name="fi"> |
The background incidence of flatulence and diarrhea was the same in patients in controlled clinical trials, and higher in the placebo group. Only constipation and dyspepsia were shown to occur in a higher percentage of patients who received Cholestagel, compared to the placebo group. Side effects were generally mild or moderate in severity. In the application of colesevelam in combination with statins, no unexpected frequent side effects occurred.<ref name="fi">{{cite web | url = http://www.genzyme.de/prod/pdf-prod/de_pdf_pt_chol_fachinfo.pdf | title = Consumer information for cholestagel | work = Genzyme | archive-url = https://web.archive.org/web/20110719031609/http://www.genzyme.de/prod/pdf-prod/de_pdf_pt_chol_fachinfo.pdf | archive-date=19 July 2011 | date = March 2009 | language = de }}</ref> |
||
== |
== References == |
||
{{reflist}} |
{{reflist}} |
||
==External links== |
|||
* {{cite web | url = https://druginfo.nlm.nih.gov/drugportal/name/colesevelam | publisher = U.S. National Library of Medicine | work = Drug Information Portal | title = Colesevelam }} |
|||
{{Lipid modifying agents}} |
{{Lipid modifying agents}} |
||
{{Portal bar | Medicine}} |
{{Portal bar | Medicine}} |
||
{{Authority control}} |
|||
[[Category:Hepatology]] |
[[Category:Hepatology]] |
||
Latest revision as of 23:22, 16 August 2024
 | |
| Clinical data | |
|---|---|
| Trade names | Welchol, Cholestagel |
| AHFS/Drugs.com | Monograph |
| MedlinePlus | a699050 |
| License data |
|
| Routes of administration | By mouth |
| ATC code | |
| Legal status | |
| Legal status | |
| Pharmacokinetic data | |
| Bioavailability | K.A. |
| Metabolism | Colesevelam is not absorbed and not metabolized |
| Elimination half-life | N/A (non-systemic drug) |
| Excretion | By intestines only, colesevelam is non-systemic |
| Identifiers | |
| |
| CAS Number | |
| PubChem CID | |
| DrugBank | |
| ChemSpider |
|
| UNII | |
| KEGG | |
| ChEMBL | |
| Chemical and physical data | |
| Formula | C31H67Cl3N4O |
| Molar mass | 618.25 g·mol−1 |
| | |
Colesevelam is a bile acid sequestrant administered orally. It was developed by GelTex Pharmaceuticals and later acquired by Genzyme. It is marketed in the US by Daiichi Sankyo under the brand name Welchol and elsewhere by Genzyme as Cholestagel. In Canada, it is marketed by Valeant as Lodalis.
Clinical use
[edit]Colesevelam is indicated as an adjunct to diet and exercise to reduce elevated low-density lipoprotein cholesterol (LDL-C) in patients with primary hyperlipidemia as monotherapy and to improve glycemic control in adults with type 2 diabetes mellitus,[4] including in combination with a statin. The expanded use of colesevelam in adults with type 2 diabetes mellitus is an example of drug repositioning.[citation needed]
Colesevelam is one of the bile-acid sequestrants, which along with niacin and the statins, are the three main types of cholesterol-lowering agents. The statins are considered the first-line agents. This is because of the larger body of evidence supporting statins' ability to prevent cardiovascular disease, as well as the prominent side effects from the other two types, including bloating and constipation (bile-acid sequestrants) and skin flushing (niacin). These side effects often lead to low patient compliance.[5]
Colesevelam can be used instead of cholestyramine in symptomatic chronic diarrhea due to bile salt malabsorption (bile acid diarrhea), which can be a primary condition, or secondary to Crohn's disease or the postcholecystectomy syndrome.[6][7][8]
Constituents
[edit]Colesevelam is a modified polyallylamine. It is made by crosslinking polyallylamine with epichlorohydrin, and then modifying it with bromodecane and (6-bromohexyl)trimethylammonium bromide. The bromide ions are then replaced with chloride ions when the material is washed.[9]
The constituents of the polymer colesevelam shown as subunits that do not exist per se in the final product are:
N-prop-2-enyldecan-1-amine; trimethyl-[6-(prop-2-enylamino)hexyl]azanium; prop-2-en-1-amine; 2-(chloromethyl)oxirane; hydrogen chloride; chloride.
Mechanism of action
[edit]Colesevelam is part of a class of drugs known as bile acid sequestrants. Colesevelam hydrochloride, the active pharmaceutical ingredient in Welchol, is a non-absorbed, lipid-lowering polymer that binds bile acids in the intestine, impeding their reabsorption. As the bile acid pool becomes depleted, the hepatic enzyme, cholesterol 7-α-hydroxylase, is upregulated, which increases the conversion of cholesterol to bile acids. This causes an increased demand for cholesterol in the liver cells, resulting in the dual effect of increasing transcription and activity of the cholesterol biosynthetic enzyme, HMG-CoA reductase, and increasing the number of hepatic LDL receptors. These compensatory effects result in increased clearance of LDL-C from the blood, resulting in decreased serum LDL-C levels. Serum TG levels may increase or remain unchanged.[10]
Side effects
[edit]In controlled clinical studies involving approximately 1,400 patients, the following adverse reactions have been reported in patients treated with colesevelam. When reporting to the very common (≥ 1 / 10), common (≥ 1 / 100, 51/10), uncommon (≥ 1 / 1000, 51/100), rare (≥ 1/10.000, 51/1000) and distinction very rarely (51/10.000), including individual cases:[citation needed]
- Investigations Common: serum triglyceride increased; Uncommon: serum transaminase increases
- Nervous system disorders Common: headache
- Gastrointestinal disorders Very Common: flatulence, constipation; Common: vomiting, diarrhea, dyspepsia, abdominal pain, stool abnormalities, nausea
- Musculoskeletal and connective tissue disorders Uncommon: myalgia
The background incidence of flatulence and diarrhea was the same in patients in controlled clinical trials, and higher in the placebo group. Only constipation and dyspepsia were shown to occur in a higher percentage of patients who received Cholestagel, compared to the placebo group. Side effects were generally mild or moderate in severity. In the application of colesevelam in combination with statins, no unexpected frequent side effects occurred.[11]
References
[edit]- ^ "Welchol- colesevelam hydrochloride tablet, film coated; Welchol- colesevelam hydrochloride for suspension". DailyMed. 7 September 2022. Archived from the original on 3 February 2023. Retrieved 16 August 2024.
- ^ "Welchol- colesevelam hydrochloride tablet, film coated; Welchol- colesevelam hydrochloride for suspension; Welchol- colesevelam hydrochloride bar, chewable". DailyMed. 10 June 2022. Archived from the original on 20 May 2024. Retrieved 16 August 2024.
- ^ "Cholestagel EPAR". European Medicines Agency (EMA). 10 March 2004. Archived from the original on 15 April 2021. Retrieved 16 August 2024.
- ^ Fonseca VA, Rosenstock J, Wang AC, Truitt KE, Jones MR (August 2008). "Colesevelam HCl improves glycemic control and reduces LDL cholesterol in patients with inadequately controlled type 2 diabetes on sulfonylurea-based therapy". Diabetes Care. 31 (8): 1479–1484. doi:10.2337/dc08-0283. PMC 2494667. PMID 18458145.
- ^ Principles and Practice of Endocrinology and Metabolism, 2000, ed. Becker, chapter 163
- ^ Puleston J, Morgan H, Andreyev J (March 2005). "New treatment for bile salt malabsorption". Gut. 54 (3): 441–442. doi:10.1136/gut.2004.054486. PMC 1774391. PMID 15711000.
- ^ Wedlake L, Thomas K, Lalji A, Anagnostopoulos C, Andreyev HJ (November 2009). "Effectiveness and tolerability of colesevelam hydrochloride for bile-acid malabsorption in patients with cancer: a retrospective chart review and patient questionnaire". Clinical Therapeutics. 31 (11): 2549–2558. doi:10.1016/j.clinthera.2009.11.027. PMID 20109999.
- ^ Beigel F, Teich N, Howaldt S, Lammert F, Maul J, Breiteneicher S, et al. (November 2014). "Colesevelam for the treatment of bile acid malabsorption-associated diarrhea in patients with Crohn's disease: a randomized, double-blind, placebo-controlled study". Journal of Crohn's & Colitis. 8 (11): 1471–1479. doi:10.1016/j.crohns.2014.05.009. PMID 24953836.
- ^ US Patent 5,607,669
- ^ "Welchol". RxList. Archived from the original on 16 January 2018. Retrieved 11 April 2009.
- ^ "Consumer information for cholestagel" (PDF). Genzyme (in German). March 2009. Archived from the original (PDF) on 19 July 2011.