2-Pyrone: Difference between revisions
Appearance
Content deleted Content added
Script assisted update of identifiers from ChemSpider, CommonChemistry and FDA for the Chem/Drugbox validation project - Updated: StdInChI StdInChIKey. |
Updating {{chembox}} (no changed fields - added verified revid - updated 'UNII_Ref', 'ChemSpiderID_Ref', 'StdInChI_Ref', 'StdInChIKey_Ref') per Chem/Drugbox validation (report [[Wikipedia_talk:Wi |
||
| Line 1: | Line 1: | ||
{{chembox |
{{chembox |
||
| verifiedrevid = 399299290 |
|||
|Reference=<ref>[http://www.sigmaaldrich.com/catalog/search/ProductDetail/ALDRICH/463159 2H-Pyran-2-one] at [[Sigma-Aldrich]]</ref> |
|Reference=<ref>[http://www.sigmaaldrich.com/catalog/search/ProductDetail/ALDRICH/463159 2H-Pyran-2-one] at [[Sigma-Aldrich]]</ref> |
||
|ImageFile=2-Pyranone.png |
|ImageFile=2-Pyranone.png |
||
| Line 6: | Line 7: | ||
|OtherNames=α-Pyrone<br>2-Pyranone<br>2''H''-Pyran-2-one |
|OtherNames=α-Pyrone<br>2-Pyranone<br>2''H''-Pyran-2-one |
||
|Section1= {{Chembox Identifiers |
|Section1= {{Chembox Identifiers |
||
| ChemSpiderID_Ref = {{chemspidercite|correct|chemspider}} |
|||
| |
| ChemSpiderID = 61462 |
||
| InChI = 1/C5H4O2/c6-5-3-1-2-4-7-5/h1-4H |
| InChI = 1/C5H4O2/c6-5-3-1-2-4-7-5/h1-4H |
||
| InChIKey = ZPSJGADGUYYRKE-UHFFFAOYAI |
| InChIKey = ZPSJGADGUYYRKE-UHFFFAOYAI |
||
| StdInChI_Ref = {{stdinchicite|correct|chemspider}} |
|||
| StdInChI = 1S/C5H4O2/c6-5-3-1-2-4-7-5/h1-4H |
| StdInChI = 1S/C5H4O2/c6-5-3-1-2-4-7-5/h1-4H |
||
| StdInChIKey_Ref = {{stdinchicite|correct|chemspider}} |
|||
| StdInChIKey = ZPSJGADGUYYRKE-UHFFFAOYSA-N |
| StdInChIKey = ZPSJGADGUYYRKE-UHFFFAOYSA-N |
||
| CASNo=504-31-4 |
| CASNo=504-31-4 |
||
Revision as of 10:16, 28 November 2010

| |
| Names | |
|---|---|
| IUPAC name
Pyran-2-one
| |
| Other names
α-Pyrone
2-Pyranone 2H-Pyran-2-one | |
| Identifiers | |
3D model (JSmol)
|
|
| ChemSpider | |
| ECHA InfoCard | 100.007.264 |
PubChem CID
|
|
CompTox Dashboard (EPA)
|
|
| |
| |
| Properties | |
| C5H4O2 | |
| Molar mass | 96.08 |
| Density | 1.197 g/mL |
| Boiling point | 102-103 °C at 20 mmHg |
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa).
| |
2-Pyrone (α-pyrone or pyran-2-one) is an unsaturated cyclic chemical compound with the molecular formula C5H4O2. It is isomeric with 4-pyrone.
2-Pyrone is used in organic synthesis as a building block for more complex chemical structures because it may participate in a variety of cycloaddition reactions to form bicyclic lactones. For example, it readily undergoes Diels-Alder reactions with alkynes producing, upon loss of carbon dioxide, substituted benzenes.[2]. The Gogte Synthesis (1938) is a method for the alkylation of certain pyrones with acid chlorides [citation needed].
-
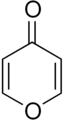
4-Pyrone
The most famous natural products are Bufanolides and Kavalactones or Kavapyrones.
References
- ^ 2H-Pyran-2-one at Sigma-Aldrich
- ^ Woodard BT, Posner G H. "Recent Advances in Diels-Alder Cycloadditions Using 2-Pyrones." Advances in Cycloaddition. 1999, 5, 47-83.