Icodextrin: Difference between revisions
Anypodetos (talk | contribs) Expand |
Anypodetos (talk | contribs) Further expansion |
||
| Line 11: | Line 11: | ||
| DrugBank = DB00702 |
| DrugBank = DB00702 |
||
| KEGG = D03266 |
| KEGG = D03266 |
||
| chemical_formula = |
| chemical_formula = (C<sub>6</sub>H<sub>10</sub>O<sub>5</sub>)<sub>n</sub> |
||
| molecular_weight = |
| molecular_weight = 13–19 [[kDa]] |
||
| smiles = |
| smiles = |
||
| bioavailability = 40% in 12 hours |
| bioavailability = 40% in 12 hours |
||
| protein_bound = |
| protein_bound = |
||
| metabolism = [[ |
| metabolism = [[Alpha-amylase]] |
||
| elimination_half-life = |
| elimination_half-life = |
||
| excretion = [[Renal]] |
| excretion = [[Renal]] |
||
| pregnancy_AU = <!-- A / B1 / B2 / B3 / C / D / X --> |
| pregnancy_AU = <!-- A / B1 / B2 / B3 / C / D / X --> |
||
| pregnancy_US = |
| pregnancy_US = C |
||
| pregnancy_category= |
|||
| legal_AU = <!-- S2, S3, S4, S5, S6, S7, S8, S9 or Unscheduled--> |
| legal_AU = <!-- S2, S3, S4, S5, S6, S7, S8, S9 or Unscheduled--> |
||
| legal_CA = <!-- Schedule I, II, III, IV, V, VI, VII, VIII --> |
| legal_CA = <!-- Schedule I, II, III, IV, V, VI, VII, VIII --> |
||
| Line 30: | Line 29: | ||
}} |
}} |
||
'''Icodextrin''' ([[International Nonproprietary Name|INN]], [[United States Adopted Name|USAN]]) is a [[colloid]] [[osmotic]] agent used in form of |
'''Icodextrin''' ([[International Nonproprietary Name|INN]], [[United States Adopted Name|USAN]]) is a [[colloid]] [[osmotic]] agent used in form of an [[aqueous]] solution for [[peritoneal dialysis]] under the trade name '''Extraneal''',<ref name="RxList" /> and after gynecological [[laparoscopic]] surgery for the reduction of post-surgical [[adhesion (medicine)|adhesion]]s (fibrous bands that form between tissues and organs) under the trade name '''Adept'''.<ref name="FDA" /> |
||
==Physical and chemical properties== |
|||
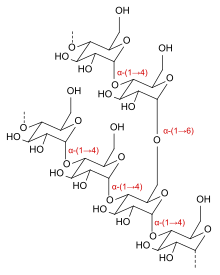
Icodextrin is a starch-derived, branched, water-soluble [[glucose]] [[polymer]] linked by α-(1-4) and less than 10% α-(1-6) [[glycosidic bond]]s with a weight-average molecular weight between 13,000 and 19,000 [[Dalton (unit)|Dalton]]s and a number-average molecular weight between 5,000 and 6,500 Daltons. The substance is a white to off-white solid, and the solution is clear and colourless to pale yellow.<ref name="FDA" /> |
|||
==Mechanism of action== |
==Mechanism of action== |
||
The osmotic activity of icodextrin keeps the solution inside the [[peritoneum]] for three to four days, reducing |
The osmotic activity of icodextrin keeps the solution inside the [[peritoneum]] for three to four days, reducing adhesion between tissues when [[fibrin]] is formed after a surgery. In other words, the tissues are kept from glueing together.<ref name="FDA" /> |
||
[[File:DP branchement.svg|thumb|left|upright|[[Peritoneal dialysis]]]] |
|||
When used for peritoneal dialysis, the icodextrin solution absorbs waste products from the blood, and is removed from the peritoneum after a few hours together with the waste.<ref>{{pmid|12962523}}</ref> |
When used for peritoneal dialysis, the icodextrin solution absorbs waste products from the blood, and is removed from the peritoneum after a few hours together with the waste.<ref>{{pmid|12962523}}</ref> |
||
==Pharmacokinetics== |
==Pharmacokinetics== |
||
Icodextrin is not metabolised inside the peritoneum. Instead, it is absorbed slowly (40% after 12 hours) into the bloodstream via the [[lymph vessel]]s. There it is broken down by the enzyme [[alpha-amylase]] |
Icodextrin is not significantly metabolised inside the peritoneum. Instead, it is absorbed slowly (40% after 12 hours) into the bloodstream via the [[lymph vessel]]s. There it is broken down into [[oligosaccharide]]s by the enzyme [[alpha-amylase]]. In patients with intact kidney function, both icodextrin and its fragments are excreted via the kidney by [[glomerular filtration]].<ref name="RxList">RxList.com: [http://www.rxlist.com/extraneal-drug.htm Extraneal]</ref><ref name="FDA">FDA: [http://www.accessdata.fda.gov/cdrh_docs/pdf5/P050011c.pdf Adept® (4% Icodextrin) Adhesion Reduction Solution]</ref> |
||
==Contraindications== |
==Contraindications== |
||
| Line 44: | Line 47: | ||
==Adverse effects== |
==Adverse effects== |
||
Adverse effects include [[peritonitis]], [[respiratory infection]], [[hypertension]] (high blood pressure), |
Adverse effects include [[peritonitis]], [[respiratory infection]], [[hypertension]] (high blood pressure), [[rash]]es, and headache. Of these side effects, only hypertension and rashes occurred significantly more often than under glucose solution; the other events seem to be related to peritoneal dialysis in general.<ref name="Drugs.com" /> |
||
==Interactions== |
==Interactions== |
||
Revision as of 18:34, 10 March 2011
 | |
| Clinical data | |
|---|---|
| Routes of administration | Intraperitoneal |
| ATC code | |
| Legal status | |
| Legal status |
|
| Pharmacokinetic data | |
| Bioavailability | 40% in 12 hours |
| Metabolism | Alpha-amylase |
| Excretion | Renal |
| Identifiers | |
| CAS Number | |
| PubChem SID | |
| DrugBank | |
| KEGG | |
| CompTox Dashboard (EPA) | |
| Chemical and physical data | |
| Formula | (C6H10O5)n |
| Molar mass | 13–19 kDa |
Icodextrin (INN, USAN) is a colloid osmotic agent used in form of an aqueous solution for peritoneal dialysis under the trade name Extraneal,[1] and after gynecological laparoscopic surgery for the reduction of post-surgical adhesions (fibrous bands that form between tissues and organs) under the trade name Adept.[2]
Physical and chemical properties
Icodextrin is a starch-derived, branched, water-soluble glucose polymer linked by α-(1-4) and less than 10% α-(1-6) glycosidic bonds with a weight-average molecular weight between 13,000 and 19,000 Daltons and a number-average molecular weight between 5,000 and 6,500 Daltons. The substance is a white to off-white solid, and the solution is clear and colourless to pale yellow.[2]
Mechanism of action
The osmotic activity of icodextrin keeps the solution inside the peritoneum for three to four days, reducing adhesion between tissues when fibrin is formed after a surgery. In other words, the tissues are kept from glueing together.[2]

When used for peritoneal dialysis, the icodextrin solution absorbs waste products from the blood, and is removed from the peritoneum after a few hours together with the waste.[3]
Pharmacokinetics
Icodextrin is not significantly metabolised inside the peritoneum. Instead, it is absorbed slowly (40% after 12 hours) into the bloodstream via the lymph vessels. There it is broken down into oligosaccharides by the enzyme alpha-amylase. In patients with intact kidney function, both icodextrin and its fragments are excreted via the kidney by glomerular filtration.[1][2]
Contraindications
Icodextrin is contraindicated in patients with cornstarch allergy, maltose or isomaltose intolerance, glycogen storage disease, or severe lactic acidosis.[4]
Adverse effects
Adverse effects include peritonitis, respiratory infection, hypertension (high blood pressure), rashes, and headache. Of these side effects, only hypertension and rashes occurred significantly more often than under glucose solution; the other events seem to be related to peritoneal dialysis in general.[4]
Interactions
Icodextrin can mimic increased blood glucose levels, depending on the used testing system. Specifically, glucose dehydrogenase pyrroloquinolinequinone (GDH-PQQ) or glucose-dye-oxidoreductase (GDO) based tests can erroneously show high blood glucose in patients that have been treated with icodextrin.[4]
