Cefoperazone: Difference between revisions
No edit summary |
Anypodetos (talk | contribs) Fix ATCvet code |
||
| Line 31: | Line 31: | ||
| ATC_prefix = J01 |
| ATC_prefix = J01 |
||
| ATC_suffix = DD12 |
| ATC_suffix = DD12 |
||
| ATC_supplemental = |
| ATC_supplemental = {{ATCvet|J51|DD12}} |
||
| PubChem = 44185 |
| PubChem = 44185 |
||
| DrugBank_Ref = {{drugbankcite|correct|drugbank}} |
| DrugBank_Ref = {{drugbankcite|correct|drugbank}} |
||
Revision as of 18:58, 25 January 2012
 | |
| Clinical data | |
|---|---|
| AHFS/Drugs.com | Micromedex Detailed Consumer Information |
| MedlinePlus | a601206 |
| ATC code | |
| Pharmacokinetic data | |
| Excretion | Hepatic |
| Identifiers | |
| |
| CAS Number | |
| PubChem CID | |
| DrugBank | |
| ChemSpider | |
| UNII | |
| KEGG | |
| ChEMBL | |
| CompTox Dashboard (EPA) | |
| ECHA InfoCard | 100.057.936 |
| Chemical and physical data | |
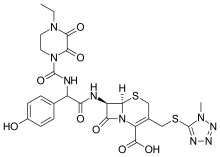
| Formula | C25H27N9O8S2 |
| Molar mass | 645.67 g/mol g·mol−1 |
| 3D model (JSmol) | |
| |
| |
| (verify) | |
Cefoperazone is a third generation cephalosporin antibiotic, marketed by Pfizer under the name Cefobid, and also marked by pharco B international under the name of Cefazone and also marketed by "sigmatec " under the name " cefoperazone" . It is one of few cephalosporin antibiotics effective in treating Pseudomonas bacterial infections which are otherwise resistant to these antibiotics.
Cefina-SB is a combination of sulbactam and cefoperazone. Cefoperazone exerts its bactericidal effect by inhibiting the bacterial cell wall synthesis, and sulbactam acts as a beta-lactamase inhibitor, to increase the antibacterial activity of cefoperazone against beta-lactamase producing organisms. In some countries, the combination is sold as Sulperazone. Gepach International markets this combination of Cefoperazone with Sulbactam under the brand name Bacperazone
Adverse effects
Cefoperazone contains an N-methylthiotetrazole (NMTT or 1-MTT) side chain. As the antibiotic is broken down in the body, it releases free NMTT, which can cause hypoprothrombinemia (likely due to inhibition of the enzyme vitamin K epoxide reductase) and a reaction with ethanol similar to that produced by disulfiram (Antabuse), due to inhibition of aldehyde dehydrogenase.[1]
References
- ^ Stork CM (2006). "Antibiotics, antifungals, and antivirals". In Nelson LH, Flomenbaum N, Goldfrank LR, Hoffman RL, Howland MD, Lewin NA (eds.) (ed.). Goldfrank's toxicologic emergencies. New York: McGraw-Hill. p. 847. ISBN 0-07-143763-0.
{{cite book}}:|access-date=requires|url=(help);|editor=has generic name (help); External link in|chapterurl=|chapterurl=ignored (|chapter-url=suggested) (help)CS1 maint: multiple names: editors list (link)
