Isocarboxazid: Difference between revisions
| Line 61: | Line 61: | ||
[[Acetonylacetone]] (not [[Acetylacetone]]) is the starting material, which on [[nitrosation]] with [[nitrous acid]] gives 5-methyl-isoxazol-3-carboxylic acid |
[[Acetonylacetone]] (not [[Acetylacetone]]) is the starting material, which on [[nitrosation]] with [[nitrous acid]] gives 5-methyl-isoxazol-3-carboxylic acid |
||
[[File:Isocarboxazid synthesis.png|thumb|center|500px|Isocarboxazid synthesis: {{US patent|2908688}} [[Hoffmann La Roche]] (1959).]] |
[[File:Isocarboxazid synthesis.png|thumb|center|500px|Isocarboxazid synthesis: {{US patent|2908688}} [[Hoffmann La Roche]] (1959).]] |
||
The requisite ester is obtained in a single step by condensation of the diketoster obtained by [[aldol condensation]] of [[acetone]] with [[diethyl oxalate]], with [[hydroxylamine]]. One explanation of the outcome of the reaction assumes the first step to consist of conjugate addition-elimination of hydroxylamine to the enolized diketone to afford an intermediate, probably in equilibrium with the enol form. |
|||
[[File:Isocarboxazid synthesis 2.svg|thumb|center|500px|Isocarboxazid synthesis 2:<ref>{{Cite doi|10.1021/jm50009a002}}</ref>]] |
[[File:Isocarboxazid synthesis 2.svg|thumb|center|500px|Isocarboxazid synthesis 2:<ref>{{Cite doi|10.1021/jm50009a002}}</ref>]] |
||
Revision as of 14:28, 9 January 2015
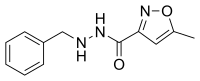 | |
| Clinical data | |
|---|---|
| Trade names | Marplan |
| AHFS/Drugs.com | Consumer Drug Information |
| MedlinePlus | a605036 |
| Pregnancy category |
|
| Routes of administration | Oral |
| ATC code | |
| Legal status | |
| Legal status | |
| Pharmacokinetic data | |
| Bioavailability | ? |
| Metabolism | Liver |
| Elimination half-life | ? |
| Excretion | Urine |
| Identifiers | |
| |
| CAS Number | |
| PubChem CID | |
| DrugBank | |
| ChemSpider | |
| UNII | |
| KEGG | |
| ChEMBL | |
| CompTox Dashboard (EPA) | |
| ECHA InfoCard | 100.000.399 |
| Chemical and physical data | |
| Formula | C12H13N3O2 |
| Molar mass | 231.25 g·mol−1 |
| 3D model (JSmol) | |
| |
| |
| | |
Isocarboxazid (Marplan, Marplon, Enerzer) is a non-selective, irreversible monoamine oxidase inhibitor (MAOI) of the hydrazine class used as an antidepressant.[2] Along with phenelzine and tranylcypromine, it is one of only three classical MAOIs still available for clinical use in the treatment of psychiatric disorders in the United States,[3][4] though it is not as commonly employed in comparison to the others.[3][4]
Isocarboxazid is primarily used to treat mood and anxiety disorders. It has also been investigated in the treatment of Parkinson's disease and other dementia-related disorders. Although efficacious, isocarboxazid produces many side effects, which may include headaches, jaundice, chest pain, weight gain, orthostatic hypotension, fainting, dizziness, and tremors. Isocarboxazid, as well as other MAOIs, increase the levels of the monoamine neurotransmitters serotonin, dopamine, and norepinephrine in the brain.[5]
Classical MAOIs, including isocarboxazid, are used only very rarely in the present day due to prominent food and drug interactions and have been largely superseded by newer, safer, and more tolerable antidepressants such as the selective serotonin reuptake inhibitors (SSRIs). The cause of the interactions is due to the fact that MAOIs inhibit the metabolism of dietary amines (e.g., tyramine) and the monoamine neurotransmitters. In combination with other drugs that increase the levels of the monoamine neurotransmitters such as the SSRIs, or with certain foods high in dietary amines such as aged cheeses, MAOIs can produce dangerous elevations of monoamine neurotransmitters resulting in potentially life-threatening syndromes such as hypertensive crisis and serotonin syndrome.
Chemistry
Isoniazide, the hydrazide of pyridine-4-carboxylic acid, is still, well over half a century after its discovery, one of the mainstays for the treatment of tuberculosis. Widespread use led to the serendipitous discovery of its antidepressant activity. This latter activity is retained when pyridine is replaced by isoxazole.
Synthesis
Acetonylacetone (not Acetylacetone) is the starting material, which on nitrosation with nitrous acid gives 5-methyl-isoxazol-3-carboxylic acid

The requisite ester is obtained in a single step by condensation of the diketoster obtained by aldol condensation of acetone with diethyl oxalate, with hydroxylamine. One explanation of the outcome of the reaction assumes the first step to consist of conjugate addition-elimination of hydroxylamine to the enolized diketone to afford an intermediate, probably in equilibrium with the enol form.

See also
References
- ^ "FDA-sourced list of all drugs with black box warnings (Use Download Full Results and View Query links.)". nctr-crs.fda.gov. FDA. Retrieved 22 Oct 2023.
- ^ Fagervall I, Ross SB (April 1986). "Inhibition of monoamine oxidase in monoaminergic neurones in the rat brain by irreversible inhibitors". Biochemical pharmacology. 35 (8): 1381–7. doi:10.1016/0006-2952(86)90285-6. PMID 2870717.
- ^ a b David Rosenberg (21 August 2013). Pocket Guide For The Textbook Of Pharmacotherapy For Child And Adolescent Psychiatric Disorders. Routledge. pp. 176–. ISBN 978-1-134-86002-9.
- ^ a b Lawrence A. Labbate; Maurizio Fava; Jerrold F. Rosenbaum (28 March 2012). Handbook of Psychiatric Drug Therapy. Lippincott Williams & Wilkins. pp. 99–. ISBN 978-1-4511-5307-1.
{{cite book}}: Unknown parameter|coauthors=ignored (|author=suggested) (help) - ^ Volz, Hanz-Peter. (November 1998) “Monoamine Oxidase Inhibitors A Perspective on Their Use in The Elderly” Biochemical pharmacology (5) 341-352.
- ^ Attention: This template ({{cite doi}}) is deprecated. To cite the publication identified by doi:10.1021/jm50009a002, please use {{cite journal}} (if it was published in a bona fide academic journal, otherwise {{cite report}} with
|doi=10.1021/jm50009a002instead.
