Boger pyridine synthesis: Difference between revisions
Appearance
Content deleted Content added
mNo edit summary |
Added tags to the page using Page Curation (refimprove, more footnotes, stub) |
||
| Line 1: | Line 1: | ||
{{Multiple issues|{{refimprove|date=October 2015}}{{more footnotes|date=October 2015}}}} |
|||
The '''Boger pyridine synthesis''' is a cycloaddition approach to the formation of [[Pyridine|pyridines]] in which an [[enamine]] reacts in an [[Inverse electron-demand Diels–Alder reaction|inverse-electron demand Diels-Alder reaction]] with a 1,2,4-[[triazine]] to form the pyridine nucleus. The reaction is especially useful for accessing pyridines that would be difficult or impossible to access via other methods. |
The '''Boger pyridine synthesis''' is a cycloaddition approach to the formation of [[Pyridine|pyridines]] in which an [[enamine]] reacts in an [[Inverse electron-demand Diels–Alder reaction|inverse-electron demand Diels-Alder reaction]] with a 1,2,4-[[triazine]] to form the pyridine nucleus. The reaction is especially useful for accessing pyridines that would be difficult or impossible to access via other methods. |
||
| Line 8: | Line 10: | ||
==References== |
==References== |
||
<ref>{{cite journal|last1=Boger|first1=D.|journal=J. Org. Chem.|date=1982|volume=47|page=895}}</ref> |
<ref>{{cite journal|last1=Boger|first1=D.|journal=J. Org. Chem.|date=1982|volume=47|page=895}}</ref> |
||
{{stub}} |
|||
Revision as of 18:50, 12 October 2015
This article has multiple issues. Please help improve it or discuss these issues on the talk page. (Learn how and when to remove these template messages)
|
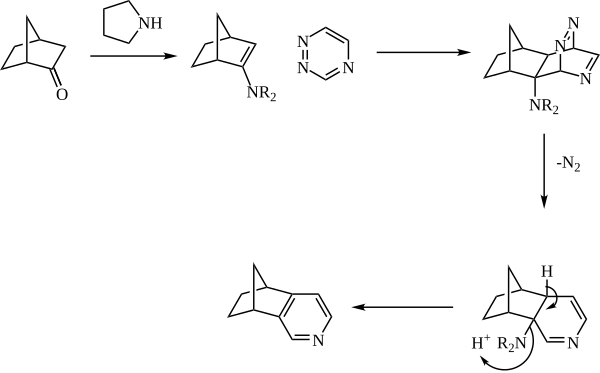
The Boger pyridine synthesis is a cycloaddition approach to the formation of pyridines in which an enamine reacts in an inverse-electron demand Diels-Alder reaction with a 1,2,4-triazine to form the pyridine nucleus. The reaction is especially useful for accessing pyridines that would be difficult or impossible to access via other methods.
Mechanism
The enamine is generally generated in situ from catalytic amine (such as pyrrolidine) and a ketone. The enamine then reacts as the dienophile with a 1,2,4-triazine. The initial adduct then expels nitrogen, and the pyridine is rearomatized with loss of the amine.
References
- ^ Boger, D. (1982). J. Org. Chem. 47: 895.
{{cite journal}}: Missing or empty|title=(help)
