Calcium iodate: Difference between revisions
more |
m Dating maintenance tags: {{Dead link}} |
||
| Line 55: | Line 55: | ||
Calcium iodate can also be used as an iodine supplement in [[chicken feed]].<ref name=Ullmann/> |
Calcium iodate can also be used as an iodine supplement in [[chicken feed]].<ref name=Ullmann/> |
||
Calcium iodate is used in the manufacture of [[disinfectant]]s, [[antiseptic]]s, and [[deodorant]]s.<ref>{{cite web | url = http://www.chemicalland21.com/lifescience/foco/CALCIUM%20IODATE.htm | title = Calcium Iodate | publisher = chemicalland21.com}}</ref><ref>[http://cancerweb.ncl.ac.uk/cgi-bin/omd?calcium+iodate Calcium iodate]{{dead link}} from the Online Medical Dictionary</ref> |
Calcium iodate is used in the manufacture of [[disinfectant]]s, [[antiseptic]]s, and [[deodorant]]s.<ref>{{cite web | url = http://www.chemicalland21.com/lifescience/foco/CALCIUM%20IODATE.htm | title = Calcium Iodate | publisher = chemicalland21.com}}</ref><ref>[http://cancerweb.ncl.ac.uk/cgi-bin/omd?calcium+iodate Calcium iodate]{{dead link|date=November 2015}} from the Online Medical Dictionary</ref> |
||
==References== |
==References== |
||
Revision as of 18:04, 30 November 2015
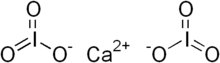
| |
| Names | |
|---|---|
| IUPAC name
Calcium diiodate
| |
| Other names
Lautarite
| |
| Identifiers | |
| |
3D model (JSmol)
|
|
| ChemSpider | |
| ECHA InfoCard | 100.029.265 |
| EC Number |
|
| E number | E916 (glazing agents, ...) |
PubChem CID
|
|
CompTox Dashboard (EPA)
|
|
| |
| |
| Properties | |
| Ca(IO3)2 | |
| Molar mass | 389.88 g/mol (anhydrous) 407.90 g/mol (monohydrate) |
| Appearance | white solid |
| Density | 4.519 g/cm3 (monohydrate) |
| Melting point | 540 °C (1,004 °F; 813 K) (monohydrate) |
| Boiling point | decomposes |
| 0.09 g/100 mL (0 °C) 0.24 g/100 mL (20 °C) 0.67 g/100 mL (90 °C) | |
| Solubility | soluble in nitric acid insoluble in alcohol |
| Structure | |
| monoclinic (anhydrous) cubic (monohydrate) orthorhombic (hexahydrate) | |
| Hazards | |
| Flash point | non-flammable |
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa).
| |
Calcium iodateis an inorganic compound composed of calcium dication and iodate anion. It is a colourless salt that occurs naturally as the mineral called lautarite, which is found in the Atacama Desert in Chile.[1]
Production and reactions
It can also be formed by the anodic oxidation of calcium iodide or by passing chlorine into a hot solution of lime in which iodine has been dissolved.
Uses
The mineral is a commercially useful precursor to iodine. Processing of the ore entails reduction of its aqueous extracts with sodium bisulfite to give sodium iodide. Via a comproportionation reaction, the sodium iodide is combined with the iodate salt to produce elemental iodine.[1]
Calcium iodate can also be used as an iodine supplement in chicken feed.[1]
Calcium iodate is used in the manufacture of disinfectants, antiseptics, and deodorants.[2][3]
References
- ^ a b c Lyday, Phyllis A. "Iodine and Iodine Compounds" in Ullmann's Encyclopedia of Industrial Chemistry, 2005, Wiley-VCH, Weinheim, ISBN 978-3-527-30673-2 doi:10.1002/14356007.a14_381 Vol. A14 pp. 382–390.
- ^ "Calcium Iodate". chemicalland21.com.
- ^ Calcium iodate[dead link] from the Online Medical Dictionary
