Multidrug resistance-associated protein 2: Difference between revisions
m Task 7c: repair/replace et al. in cs1 author/editor parameters; |
|||
| Line 1: | Line 1: | ||
{{PBB|geneid=1244}} |
{{PBB|geneid=1244}} |
||
'''Multidrug resistance-associated protein 2''' (MRP2) also called '''canalicular multispecific organic anion transporter 1''' (cMOAT) or '''ATP-binding cassette sub-family C member 2''' (ABCC2) is a [[protein]] that in humans is encoded by the ''ABCC2'' [[gene]].<ref name="pmid8797578"/><ref name="pmid9284939"/><ref name="entrez"/> |
'''Multidrug resistance-associated protein 2''' (MRP2) also called '''canalicular multispecific organic anion transporter 1''' (cMOAT) or '''ATP-binding cassette sub-family C member 2''' (ABCC2) is a [[protein]] that in humans is encoded by the ''ABCC2'' [[gene]].<ref name="pmid8797578"/><ref name="pmid9284939"/><ref name="entrez"/> |
||
== Function == |
== Function == |
||
MRP2 is a member of the superfamily of [[ATP-binding cassette transporter|ATP-binding cassette (ABC) transporters]]. ABC proteins transport various molecules across extra- and intra-[[cellular membrane]]s. ABC genes are divided into seven distinct subfamilies (ABC1, MDR/TAP, MRP, ALD, OABP, GCN20, White). More specifically, this protein is a member of the MRP subfamily, which is involved in [[multi-drug resistance]]. This protein is expressed in the canalicular (apical) part of the [[hepatocyte]] and functions in biliary transport. Substrates include [[chemotherapy|anticancer drug]]s such as [[vinblastine]]; therefore, this protein appears to contribute to drug resistance in mammalian cells. |
MRP2 is a member of the superfamily of [[ATP-binding cassette transporter|ATP-binding cassette (ABC) transporters]]. ABC proteins transport various molecules across extra- and intra-[[cellular membrane]]s. ABC genes are divided into seven distinct subfamilies (ABC1, MDR/TAP, MRP, ALD, OABP, GCN20, White). More specifically, this protein is a member of the MRP subfamily, which is involved in [[multi-drug resistance]]. This protein is expressed in the canalicular (apical) part of the [[hepatocyte]] and functions in biliary transport. Substrates include [[chemotherapy|anticancer drug]]s such as [[vinblastine]]; therefore, this protein appears to contribute to drug resistance in mammalian cells. |
||
MRP2 is also expressed in on the [[Epithelial polarity#Apical membranes|apical membrane]] of [[proximal renal tubule]] [[endothelial cells]] where they are involved in the excretion of small [[ion|organic anions]].<ref name="pmid16403838">{{cite journal | author = Sekine T, Miyazaki H, Endou H | title = Molecular physiology of renal organic anion transporters | journal = Am. J. Physiol. Renal Physiol. | volume = 290 | issue = 2 | pages = F251–61 |date=February 2006 | pmid = 16403838 | doi = 10.1152/ajprenal.00439.2004 | url = | issn = }}</ref>₳ |
MRP2 is also expressed in on the [[Epithelial polarity#Apical membranes|apical membrane]] of [[proximal renal tubule]] [[endothelial cells]] where they are involved in the excretion of small [[ion|organic anions]].<ref name="pmid16403838">{{cite journal | author = Sekine T, Miyazaki H, Endou H | title = Molecular physiology of renal organic anion transporters | journal = Am. J. Physiol. Renal Physiol. | volume = 290 | issue = 2 | pages = F251–61 |date=February 2006 | pmid = 16403838 | doi = 10.1152/ajprenal.00439.2004 | url = | issn = }}</ref>₳ |
||
| Line 22: | Line 21: | ||
| [[gout]] <br/> [[hyperuricemia]] |
| [[gout]] <br/> [[hyperuricemia]] |
||
| <ref name="pmid10727523"/> |
| <ref name="pmid10727523"/> |
||
| [[image:Probenecid.svg|| |100px]] |
| [[image:Probenecid.svg | | |100px]] |
||
|- |
|- |
||
| [[furosemide]] |
| [[furosemide]] |
||
| Line 28: | Line 27: | ||
| [[heart failure]] <br/> [[edema]] |
| [[heart failure]] <br/> [[edema]] |
||
| <ref name="pmid10727523"/> |
| <ref name="pmid10727523"/> |
||
| [[image:Furosemide.svg|| |100px]] |
| [[image:Furosemide.svg | | |100px]] |
||
|- |
|- |
||
| [[ritonavir]] |
| [[ritonavir]] |
||
| Line 34: | Line 33: | ||
| [[antiretroviral]] |
| [[antiretroviral]] |
||
| <ref name="pmid15076241"/> |
| <ref name="pmid15076241"/> |
||
| [[image:Ritonavir.svg|| |100px]] |
| [[image:Ritonavir.svg | | |100px]] |
||
|- |
|- |
||
| [[saquinavir]] |
| [[saquinavir]] |
||
| Line 40: | Line 39: | ||
| [[antiretroviral]] |
| [[antiretroviral]] |
||
| <ref name="pmid15167283"/> |
| <ref name="pmid15167283"/> |
||
| [[image:Saquinavir.svg|| |100px]] |
| [[image:Saquinavir.svg | | |100px]] |
||
|- |
|- |
||
| [[lamivudine]] |
| [[lamivudine]] |
||
| Line 46: | Line 45: | ||
| [[Antiviral drug|antiviral]] |
| [[Antiviral drug|antiviral]] |
||
| <ref name="pmid17172311"/> |
| <ref name="pmid17172311"/> |
||
| [[image:lamivudine.svg|| |100px]] |
| [[image:lamivudine.svg | | |100px]] |
||
|- |
|- |
||
| [[abacavir]] |
| [[abacavir]] |
||
| Line 52: | Line 51: | ||
| [[antiretroviral]] |
| [[antiretroviral]] |
||
| <ref name="pmid17172311"/> |
| <ref name="pmid17172311"/> |
||
| [[image:Abacavir.svg|| |100px]] |
| [[image:Abacavir.svg| | |100px]] |
||
|- |
|- |
||
| [[emtricitabine]] |
| [[emtricitabine]] |
||
| Line 58: | Line 57: | ||
| [[Antiviral drug|antiviral]] |
| [[Antiviral drug|antiviral]] |
||
| <ref name="pmid17172311"/> |
| <ref name="pmid17172311"/> |
||
| [[image:Emtricitabine skeletal.svg|| |100px]] |
| [[image:Emtricitabine skeletal.svg | | |100px]] |
||
|- |
|- |
||
| [[efavirenz]] |
| [[efavirenz]] |
||
| Line 64: | Line 63: | ||
| [[antiretroviral]] |
| [[antiretroviral]] |
||
| <ref name="pmid17172311"/> |
| <ref name="pmid17172311"/> |
||
| [[image:Efavirenz.svg|| |100px]] |
| [[image:Efavirenz.svg | | |100px]] |
||
|- |
|- |
||
| [[delavirdine]] |
| [[delavirdine]] |
||
| Line 70: | Line 69: | ||
| [[antiretroviral]] |
| [[antiretroviral]] |
||
| <ref name="pmid17172311"/> |
| <ref name="pmid17172311"/> |
||
| [[image:Delavirdine.svg|| |100px]] |
| [[image:Delavirdine.svg | | |100px]] |
||
|- |
|- |
||
| [[nevirapine]] |
| [[nevirapine]] |
||
| Line 76: | Line 75: | ||
| [[antiretroviral]] |
| [[antiretroviral]] |
||
| <ref name="pmid17172311"/> |
| <ref name="pmid17172311"/> |
||
| [[image:Nevirapine.svg|| |100px]] |
| [[image:Nevirapine.svg | | |100px]] |
||
|- |
|- |
||
| [[cidofovir]] |
| [[cidofovir]] |
||
| Line 82: | Line 81: | ||
| [[Antiviral drug|antiviral]] |
| [[Antiviral drug|antiviral]] |
||
| <ref name="pmid11602668"/> |
| <ref name="pmid11602668"/> |
||
| [[image:Cidofovir.svg|| |100px]] |
| [[image:Cidofovir.svg | | |100px]] |
||
|- |
|- |
||
| [[adefovir]] |
| [[adefovir]] |
||
| Line 88: | Line 87: | ||
| [[Antiviral drug|antiviral]] |
| [[Antiviral drug|antiviral]] |
||
| <ref name="pmid15167283"/> |
| <ref name="pmid15167283"/> |
||
| [[image:Adefovir.svg|| |100px]] |
| [[image:Adefovir.svg | | |100px]] |
||
|- |
|- |
||
| [[tenofovir]] |
| [[tenofovir]] |
||
| Line 94: | Line 93: | ||
| [[Antiviral drug|antiviral]] |
| [[Antiviral drug|antiviral]] |
||
| <ref name="pmid17172311"/> |
| <ref name="pmid17172311"/> |
||
| [[image:Tenofovir.svg|| |100px]] |
| [[image:Tenofovir.svg | | |100px]] |
||
|- |
|- |
||
|} |
|} |
||
== Clinical significance == |
== Clinical significance == |
||
=== Dubin-Johnson syndrome === |
=== Dubin-Johnson syndrome === |
||
| Line 106: | Line 104: | ||
=== Iatrogenic Fanconi syndrome === |
=== Iatrogenic Fanconi syndrome === |
||
Many negatively charged metabolic waste products are eliminated from the body by the kidneys. These [[organic anion]]s are transported from the blood into the [[endothelial]] cells of the [[proximal convoluted tubule|renal proximal tubules]] by the [[OAT1]] transporter. From there, these waste [[molecules]] are transported into the [[Lumen (anatomy)|lumen]] of the tubule by the MRP2 transporter. Many drugs are eliminated from the body by this mechanism. Some of these drugs pass through the MRP2 transporter slowly. This may cause a |
Many negatively charged metabolic waste products are eliminated from the body by the kidneys. These [[organic anion]]s are transported from the blood into the [[endothelial]] cells of the [[proximal convoluted tubule|renal proximal tubules]] by the [[OAT1]] transporter. From there, these waste [[molecules]] are transported into the [[Lumen (anatomy)|lumen]] of the tubule by the MRP2 transporter. Many drugs are eliminated from the body by this mechanism. Some of these drugs pass through the MRP2 transporter slowly. This may cause a build up of organic anions in the [[cytoplasm]] of the cells. |
||
Drugs that inhibit the MRP2 transporter can cause a |
Drugs that inhibit the MRP2 transporter can cause a build up of organic anions inside renal proximal tubule cells. If some of these organic anions inhibit mitochondrial DNA synthesis, it may cause [[OAT1#Antiviral induced Fanconi syndrome|iatrogenic Fanconi syndrome]]. The nucleoside phosphonate [[adefovir]] is a MRP2 inhibitor that has been linked to kidney disease.<ref name="pmid12606735"/> [[Tenofovir]]<ref name = "pmid19334329"/> and [[cidofovir]]<ref name = "pmid9257778"/> are also nucleoside phosphonates that inhibit MRP2 and have been associated with Fanconi syndrome. |
||
==Interactive pathway map== |
==Interactive pathway map== |
||
| Line 117: | Line 115: | ||
==References== |
==References== |
||
{{reflist | refs = |
{{reflist | refs = |
||
<ref name="pmid8797578">{{cite journal | author = Taniguchi K, Wada M, Kohno K, Nakamura T, Kawabe T, Kawakami M, Kagotani K, Okumura K, Akiyama S, Kuwano M | title = A human canalicular multispecific organic anion transporter (cMOAT) gene is overexpressed in cisplatin-resistant human cancer cell lines with decreased drug accumulation | journal = Cancer Res | volume = 56 | issue = 18 | pages = 4124–9 |date=Oct 1996 | pmid = 8797578 | pmc = | doi = | url = http://cancerres.aacrjournals.org/content/56/18/4124.long }}</ref> |
<ref name="pmid8797578">{{cite journal | author = Taniguchi K, Wada M, Kohno K, Nakamura T, Kawabe T, Kawakami M, Kagotani K, Okumura K, Akiyama S, Kuwano M | title = A human canalicular multispecific organic anion transporter (cMOAT) gene is overexpressed in cisplatin-resistant human cancer cell lines with decreased drug accumulation | journal = Cancer Res | volume = 56 | issue = 18 | pages = 4124–9 |date=Oct 1996 | pmid = 8797578 | pmc = | doi = | url = http://cancerres.aacrjournals.org/content/56/18/4124.long }}</ref> |
||
| Line 144: | Line 142: | ||
| url = http://meta.wkhealth.com/pt/pt-core/template-journal/lwwgateway/media/landingpage.htm?issn=1525-4135&volume=35&issue=3&spage=269 |
| url = http://meta.wkhealth.com/pt/pt-core/template-journal/lwwgateway/media/landingpage.htm?issn=1525-4135&volume=35&issue=3&spage=269 |
||
| issn = |
| issn = |
||
}}</ref> |
}}</ref> |
||
<ref name="pmid15167283">{{cite journal |
<ref name="pmid15167283">{{cite journal |
||
| Line 158: | Line 156: | ||
| url = http://meta.wkhealth.com/pt/pt-core/template-journal/lwwgateway/media/landingpage.htm?issn=1525-4135&volume=36&issue=2&spage=649 |
| url = http://meta.wkhealth.com/pt/pt-core/template-journal/lwwgateway/media/landingpage.htm?issn=1525-4135&volume=36&issue=2&spage=649 |
||
| issn = |
| issn = |
||
}}</ref> |
}}</ref> |
||
<ref name="pmid11602668">{{cite journal |
<ref name="pmid11602668">{{cite journal |
||
| Line 172: | Line 170: | ||
| url = http://jpet.aspetjournals.org/cgi/pmidlookup?view=long&pmid=11602668 |
| url = http://jpet.aspetjournals.org/cgi/pmidlookup?view=long&pmid=11602668 |
||
| issn = |
| issn = |
||
}}</ref> |
}}</ref> |
||
<ref name="pmid10727523">{{cite journal |
<ref name="pmid10727523">{{cite journal |
||
| Line 186: | Line 184: | ||
| url = http://molpharm.aspetjournals.org/cgi/pmidlookup?view=long&pmid=10727523 |
| url = http://molpharm.aspetjournals.org/cgi/pmidlookup?view=long&pmid=10727523 |
||
| issn = |
| issn = |
||
}}</ref> |
}}</ref> |
||
}} |
}} |
||
Revision as of 18:32, 14 December 2015
Template:PBB Multidrug resistance-associated protein 2 (MRP2) also called canalicular multispecific organic anion transporter 1 (cMOAT) or ATP-binding cassette sub-family C member 2 (ABCC2) is a protein that in humans is encoded by the ABCC2 gene.[1][2][3]
Function
MRP2 is a member of the superfamily of ATP-binding cassette (ABC) transporters. ABC proteins transport various molecules across extra- and intra-cellular membranes. ABC genes are divided into seven distinct subfamilies (ABC1, MDR/TAP, MRP, ALD, OABP, GCN20, White). More specifically, this protein is a member of the MRP subfamily, which is involved in multi-drug resistance. This protein is expressed in the canalicular (apical) part of the hepatocyte and functions in biliary transport. Substrates include anticancer drugs such as vinblastine; therefore, this protein appears to contribute to drug resistance in mammalian cells.
MRP2 is also expressed in on the apical membrane of proximal renal tubule endothelial cells where they are involved in the excretion of small organic anions.[4]₳
MRP2 inhibitors
| Drug | Class | Indications | Source | Structure |
|---|---|---|---|---|
| probenecid | uricosuric | gout hyperuricemia |
[5] | 
|
| furosemide | loop diuretic | heart failure edema |
[5] | 
|
| ritonavir | protease inhibitor | antiretroviral | [6] | 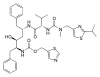
|
| saquinavir | protease inhibitor | antiretroviral | [7] | 
|
| lamivudine | Nucleoside analog | antiviral | [8] | 
|
| abacavir | Nucleoside analog | antiretroviral | [8] | 
|
| emtricitabine | Nucleoside analog | antiviral | [8] | 
|
| efavirenz | NNRTI | antiretroviral | [8] | 
|
| delavirdine | NNRTI | antiretroviral | [8] | |
| nevirapine | NNRTI | antiretroviral | [8] | 
|
| cidofovir | nucleoside phosphonate | antiviral | [9] | 
|
| adefovir | nucleoside phosphonate | antiviral | [7] | |
| tenofovir | nucleoside phosphonate | antiviral | [8] | 
|
Clinical significance
Dubin-Johnson syndrome
Several different mutations in this gene have been observed in patients with Dubin-Johnson syndrome (DJS), an autosomal recessive disorder characterized by conjugated hyperbilirubinemia.[3]
Iatrogenic Fanconi syndrome
Many negatively charged metabolic waste products are eliminated from the body by the kidneys. These organic anions are transported from the blood into the endothelial cells of the renal proximal tubules by the OAT1 transporter. From there, these waste molecules are transported into the lumen of the tubule by the MRP2 transporter. Many drugs are eliminated from the body by this mechanism. Some of these drugs pass through the MRP2 transporter slowly. This may cause a build up of organic anions in the cytoplasm of the cells.
Drugs that inhibit the MRP2 transporter can cause a build up of organic anions inside renal proximal tubule cells. If some of these organic anions inhibit mitochondrial DNA synthesis, it may cause iatrogenic Fanconi syndrome. The nucleoside phosphonate adefovir is a MRP2 inhibitor that has been linked to kidney disease.[10] Tenofovir[11] and cidofovir[12] are also nucleoside phosphonates that inhibit MRP2 and have been associated with Fanconi syndrome.
Interactive pathway map
Click on genes, proteins and metabolites below to link to respective articles. [§ 1]
- ^ The interactive pathway map can be edited at WikiPathways: "IrinotecanPathway_WP229".
See also
References
- ^ Taniguchi K, Wada M, Kohno K, Nakamura T, Kawabe T, Kawakami M, Kagotani K, Okumura K, Akiyama S, Kuwano M (Oct 1996). "A human canalicular multispecific organic anion transporter (cMOAT) gene is overexpressed in cisplatin-resistant human cancer cell lines with decreased drug accumulation". Cancer Res. 56 (18): 4124–9. PMID 8797578.
{{cite journal}}: CS1 maint: multiple names: authors list (link) - ^ van Kuijck MA, Kool M, Merkx GF, Geurts van Kessel A, Bindels RJ, Deen PM, van Os CH (Sep 1997). "Assignment of the canalicular multispecific organic anion transporter gene (CMOAT) to human chromosome 10q24 and mouse chromosome 19D2 by fluorescent in situ hybridization". Cytogenet Cell Genet. 77 (3–4): 285–7. doi:10.1159/000134599. PMID 9284939.
{{cite journal}}: CS1 maint: multiple names: authors list (link) - ^ a b "Entrez Gene: ABCC2 ATP-binding cassette, sub-family C (CFTR/MRP), member 2".
- ^ Sekine T, Miyazaki H, Endou H (February 2006). "Molecular physiology of renal organic anion transporters". Am. J. Physiol. Renal Physiol. 290 (2): F251–61. doi:10.1152/ajprenal.00439.2004. PMID 16403838.
{{cite journal}}: CS1 maint: multiple names: authors list (link) - ^ a b Bakos E, Evers R, Sinkó E, Váradi A, Borst P, Sarkadi B (April 2000). "Interactions of the human multidrug resistance proteins MRP1 and MRP2 with organic anions". Mol. Pharmacol. 57 (4): 760–8. PMID 10727523.
{{cite journal}}: CS1 maint: multiple names: authors list (link) - ^ Peyrière H, Reynes J, Rouanet I, et al. (March 2004). "Renal tubular dysfunction associated with tenofovir therapy: report of 7 cases". J. Acquir. Immune Defic. Syndr. 35 (3): 269–73. PMID 15076241.
- ^ a b Gimenez F, Fernandez C, Mabondzo A (June 2004). "Transport of HIV protease inhibitors through the blood–brain barrier and interactions with the efflux proteins, P-glycoprotein and multidrug resistance proteins". J. Acquir. Immune Defic. Syndr. 36 (2): 649–58. doi:10.1097/00126334-200406010-00001. PMID 15167283.
{{cite journal}}: CS1 maint: multiple names: authors list (link) - ^ a b c d e f g Weiss J, Theile D, Ketabi-Kiyanvash N, Lindenmaier H, Haefeli WE (March 2007). "Inhibition of MRP1/ABCC1, MRP2/ABCC2, and MRP3/ABCC3 by nucleoside, nucleotide, and non-nucleoside reverse transcriptase inhibitors". Drug Metab. Dispos. 35 (3): 340–4. doi:10.1124/dmd.106.012765. PMID 17172311.
{{cite journal}}: CS1 maint: multiple names: authors list (link) - ^ Miller DS (November 2001). "Nucleoside phosphonate interactions with multiple organic anion transporters in renal proximal tubule". J. Pharmacol. Exp. Ther. 299 (2): 567–74. PMID 11602668.
- ^ Marcellin P, Chang TT, Lim SG, et al. (February 2003). "Adefovir dipivoxil for the treatment of hepatitis B e antigen-positive chronic hepatitis B". N. Engl. J. Med. 348 (9): 808–16. doi:10.1056/NEJMoa020681. PMID 12606735.
- ^ Atta MG, Fine DM (March 2009). "Editorial comment: tenofovir nephrotoxicity--the disconnect between clinical trials and real-world practice". AIDS Read. 19 (3): 118–9. PMID 19334329.
- ^ Vittecoq D, Dumitrescu L, Beaufils H, Deray G (August 1997). "Fanconi syndrome associated with cidofovir therapy". Antimicrob. Agents Chemother. 41 (8): 1846. PMC 164022. PMID 9257778.
{{cite journal}}: CS1 maint: multiple names: authors list (link)
Further reading
- Mallants R, Van Oosterwyck K, Van Vaeck L, Mols R, De Clercq E, Augustijns P (2005). "Multidrug resistance-associated protein 2 (MRP2) affects hepatobiliary elimination but not the intestinal disposition of tenofovir disoproxil fumarate and its metabolites". Xenobiotica. 35 (10–11): 1055–66. doi:10.1080/00498250500354493. PMID 16393861.
{{cite journal}}: CS1 maint: multiple names: authors list (link)
External links
- ABCC2+protein,+human at the U.S. National Library of Medicine Medical Subject Headings (MeSH)
This article incorporates text from the United States National Library of Medicine, which is in the public domain.

