Phentolamine: Difference between revisions
m refs using AWB |
Cpt ricard (talk | contribs) →Uses: prose, actualization, removal of redundant sentence. |
||
| Line 63: | Line 63: | ||
Phentolamine also has diagnostic and therapeutic roles in [[complex regional pain syndrome]] (reflex sympathetic dystrophy).<ref>Rowbotham MC. Pharmacologic management of complex regional pain syndrome. ''Clinical Journal of Pain''. 2006 Jun;22(5):425-9.</ref> |
Phentolamine also has diagnostic and therapeutic roles in [[complex regional pain syndrome]] (reflex sympathetic dystrophy).<ref>Rowbotham MC. Pharmacologic management of complex regional pain syndrome. ''Clinical Journal of Pain''. 2006 Jun;22(5):425-9.</ref> |
||
Phentolamine |
Phentolamine is marketed in the dental field as a local anesthetic reversal agent. Marketed as OraVerse, it is a phentolamine mesylate injection designed to reverse the local vasoconstrictor properties used in many local anesthetics to prolong anesthesia.<ref>http://www.novalar.com/oraverse-dental-specialty-pharmaceutical</ref> <ref>Malamed S. What's new in local anaesthesia? ''Society For The Advancement Of Anaesthesia In Dentistry Digest''. 2009 Jan;25:4-14.</ref> |
||
==Chemistry== |
==Chemistry== |
||
Revision as of 23:51, 29 June 2016
 | |
 | |
| Clinical data | |
|---|---|
| Trade names | Regitine |
| AHFS/Drugs.com | Micromedex Detailed Consumer Information |
| Pregnancy category |
|
| Routes of administration | Usually IV or IM |
| ATC code | |
| Pharmacokinetic data | |
| Metabolism | Hepatic |
| Elimination half-life | 19 minutes |
| Identifiers | |
| |
| CAS Number | |
| PubChem CID | |
| IUPHAR/BPS | |
| DrugBank | |
| ChemSpider | |
| UNII | |
| KEGG | |
| ChEBI | |
| ChEMBL | |
| CompTox Dashboard (EPA) | |
| ECHA InfoCard | 100.000.049 |
| Chemical and physical data | |
| Formula | C17H19N3O |
| Molar mass | 281.352 g/mol g·mol−1 |
| 3D model (JSmol) | |
| |
| |
| | |
Phentolamine (Regitine) is a reversible[1] nonselective alpha-adrenergic antagonist.[2]
Mechanism
Its primary action is vasodilation due to α1 blockade.[3]
Non-selective α-blockers can cause a much more pronounced reflex tachycardia than the selective α-1 blockers. Like the selective α-1 blockers, phentolamine causes a relaxation of systemic vasculature, leading to hypotension. This hypotension is sensed by the baroreceptor reflex, which results in increased sympathetic nerve firing on the heart, releasing norepinephrine. In response, the β-1 adrenergic receptors on the heart increase its rate, contractility, and dromotropy, which help to offset the decrease in systemic blood pressure. Unlike the α-1 selective blockers, phentolamine also inhibits the α2 receptors, which function predominantly as presynaptic negative feedback for norepinephrine release. By abolishing this negative feedback phentolamine leads to even less regulated norepinephrine release, which results in a more drastic increase in heart rate.[4]
Uses
The primary application for phentolamine is for the control of hypertensive emergencies, most notably due to pheochromocytoma.[5]
It also has usefulness in the treatment of cocaine-induced cardiovascular complications, where one would generally avoid Beta-blockers (e.g. metoprolol), as they can cause unopposed alpha-adrenergic mediated coronary vasoconstriction, worsening myocardial ischemia and hypertension. It is important to note that phentolamine is not a first-line agent for this indication. Phentolamine should only be given to patients who do not fully respond to benzodiazepines, nitroglycerin, and calcium channel blockers.[6][7]
When given by injection it causes blood vessels to dilate, thereby increasing blood flow. When injected into the penis (intracavernosal), it increases blood flow to the penis, which results in an erection.[8]
It may be stored in crash carts to counteract severe peripheral vasoconstriction secondary to extravasation of peripherally placed vasopressor infusions, typically of norepinephrine. Epinephrine infusions are less vasoconstrictive than norepinephrine as they primarily stimulate beta receptor more than alpha receptors, but the effect remains dose dependent.
Phentolamine also has diagnostic and therapeutic roles in complex regional pain syndrome (reflex sympathetic dystrophy).[9]
Phentolamine is marketed in the dental field as a local anesthetic reversal agent. Marketed as OraVerse, it is a phentolamine mesylate injection designed to reverse the local vasoconstrictor properties used in many local anesthetics to prolong anesthesia.[10] [11]
Chemistry
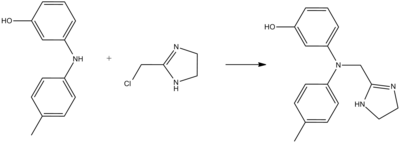
Phentolamine can be synthesized by alkylation of 3-(4-methylanilino)phenol using 2-chloromethylimidazoline:[12][13]
References
- ^ Jewell, John R.; Longworth, David L.; Stoller, James K.; Casey, David (2003). The Cleveland Clinic internal medicine case reviews. Hagerstown, MD: Lippincott Williams & Wilkins. p. 32. ISBN 0-7817-4266-8.
- ^ Phentolamine at the U.S. National Library of Medicine Medical Subject Headings (MeSH)
- ^ Brock G. Oral phentolamine (Vasomax). Drugs Today (Barcelona). 2000 Feb-Mar;36(2-3):121-4.
- ^ Shen, Howard (2008). Illustrated Pharmacology Memory Cards: PharMnemonics. Minireview. p. 14. ISBN 1-59541-101-1.
- ^ Tuncel M, Ram VC. Hypertensive emergencies. Etiology and management. American Journal of Cardiovascular Drugs. 2003;3(1):21-31.
- ^ Hollander JE, Henry TD. Evaluation and management of the patient who has cocaine-associated chest pain. Cardiology Clinics. 2006 Feb;24(1):103-14.
- ^ Chan GM, Sharma R, Price D, Hoffman RS, Nelson LS. Phentolamine Therapy for Cocaine-Association Acute Coronary Syndrome (CAACS). Journal of Medical Toxicology. 2006 Sep;2(3):108-11.
- ^ Bella AJ, Brock GB. Intracavernous pharmacotherapy for erectile dysfunction. Endocrine. 2004 Mar-Apr;23(2-3):149-55.
- ^ Rowbotham MC. Pharmacologic management of complex regional pain syndrome. Clinical Journal of Pain. 2006 Jun;22(5):425-9.
- ^ http://www.novalar.com/oraverse-dental-specialty-pharmaceutical
- ^ Malamed S. What's new in local anaesthesia? Society For The Advancement Of Anaesthesia In Dentistry Digest. 2009 Jan;25:4-14.
- ^ K. Miescher, A. Marxer, E. Urech, U.S. patent 2,503,059 (1950)
- ^ E. Urech, A. Marxer, K. Miescher, Helv. Chim. Acta, 33, 1386 (1950)