FOX-7: Difference between revisions
Rescuing 1 sources and tagging 0 as dead. #IABot (v1.5.4) |
Corrected 2 references which were to a secondary source rather than the original primary articles. |
||
| Line 35: | Line 35: | ||
}} |
}} |
||
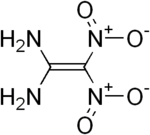
'''FOX-7''' or '''1,1-diamino-2,2-dinitroethene''' '''(DADNE)'''<ref>US Patent 6340780 - Method of preparing salts of dinitromethane</ref> is an [[Insensitive munition#Insensitive high explosives|insensitive high explosive]] compound. It was first synthesized in 1998 by the [[Swedish National Defence Research Institute]] (FOA).<ref name="dsto"> |
'''FOX-7''' or '''1,1-diamino-2,2-dinitroethene''' '''(DADNE)'''<ref>US Patent 6340780 - Method of preparing salts of dinitromethane</ref> is an [[Insensitive munition#Insensitive high explosives|insensitive high explosive]] compound. It was first synthesized in 1998 by the [[Swedish National Defence Research Institute]] (FOA).<ref name="dsto">Bemm, U.; Östmark, H. (1998) "1,1-Diamino-2,2-dinitroethylene: a Novel Energetic Material with Infinite Layers in Two Dimensions." ''Acta Crys''t '''C54''': 1–3. [[doi:10.1107/S0108270198007987]]</ref> |
||
FOX-7 is similar to the insensitive chemical compound [[TATB]], which is a [[benzene]] ring compound with three [[amino]] and three [[nitro group]]s.<ref>{{cite journal|doi=10.1016/j.tet.2005.05.010|title=The reactivity of 1,1-diamino-2,2-dinitroethene (FOX-7)|year=2005|last1=Hervé|first1=Grégoire|last2=Jacob|first2=Guy|last3=Latypov|first3=Nikolaj|journal=Tetrahedron|volume=61|issue=28|pages=6743}}</ref> FOX-7 has a two-carbon backbone rather than a [[benzene]] ring, but the [[amino]] and [[nitro compound|nitro]] groups have similar effects in both cases according to published reports on sensitivity and chemical decay processes of FOX-7. FOX-7 is today produced by Eureco [[Bofors]] AB in Sweden.<ref>{{cite journal|doi=10.1016/j.jhazmat.2006.03.034|title=Synthesis, characterization and thermolysis of 1,1-diamino-2,2-dinitroethylene (FOX-7) and its salts|year=2006|last1=Anniyappan|first1=M.|last2=Talawar|first2=M.B.|last3=Gore|first3=G.M.|last4=Venugopalan|first4=S.|last5=Gandhe|first5=B.R.|journal=Journal of Hazardous Materials|volume=137|issue=2|pages=812–9|pmid=16701943}}</ref> |
FOX-7 is similar to the insensitive chemical compound [[TATB]], which is a [[benzene]] ring compound with three [[amino]] and three [[nitro group]]s.<ref>{{cite journal|doi=10.1016/j.tet.2005.05.010|title=The reactivity of 1,1-diamino-2,2-dinitroethene (FOX-7)|year=2005|last1=Hervé|first1=Grégoire|last2=Jacob|first2=Guy|last3=Latypov|first3=Nikolaj|journal=Tetrahedron|volume=61|issue=28|pages=6743}}</ref> FOX-7 has a two-carbon backbone rather than a [[benzene]] ring, but the [[amino]] and [[nitro compound|nitro]] groups have similar effects in both cases according to published reports on sensitivity and chemical decay processes of FOX-7. FOX-7 is today produced by Eureco [[Bofors]] AB in Sweden.<ref>{{cite journal|doi=10.1016/j.jhazmat.2006.03.034|title=Synthesis, characterization and thermolysis of 1,1-diamino-2,2-dinitroethylene (FOX-7) and its salts|year=2006|last1=Anniyappan|first1=M.|last2=Talawar|first2=M.B.|last3=Gore|first3=G.M.|last4=Venugopalan|first4=S.|last5=Gandhe|first5=B.R.|journal=Journal of Hazardous Materials|volume=137|issue=2|pages=812–9|pmid=16701943}}</ref> |
||
Its explosive properties appear extremely favorable; in addition to its insensitive properties, the detonation velocity of mixtures of 80% FOX-7 plus [[binder (material)|binders]] is as high as [[Composition B]], and nearly pure FOX-7 based [[plastic bonded explosive]]s are slightly superior to [[RDX]]. |
Its explosive properties appear extremely favorable; in addition to its insensitive properties, the detonation velocity of mixtures of 80% FOX-7 plus [[binder (material)|binders]] is as high as [[Composition B]], and nearly pure FOX-7 based [[plastic bonded explosive]]s are slightly superior to [[RDX]]. |
||
<ref>[http://www.intdetsymp.org/detsymp2002/PaperSubmit/FinalManuscript/pdf/Karlsson-165.pdf DETONATION AND SENSITIVITY PROPERTIES OF FOX-7 AND FORMULATIONS CONTAINING FOX-7], Karlsson et al., 2002, accessed Aug 25, 2005</ref> FOX-7 has been calculated to have a [[detonation velocity]] of 8,870 m/s.<ref |
<ref>[http://www.intdetsymp.org/detsymp2002/PaperSubmit/FinalManuscript/pdf/Karlsson-165.pdf DETONATION AND SENSITIVITY PROPERTIES OF FOX-7 AND FORMULATIONS CONTAINING FOX-7], Karlsson et al., 2002, accessed Aug 25, 2005</ref> FOX-7 has been calculated to have a [[detonation velocity]] of 8,870 m/s.<ref>{{Cite journal|last=Latypov|first=Nikolai V.|last2=Bergman|first2=Jan|last3=Langlet|first3=Abraham|last4=Wellmar|first4=Ulf|last5=Bemm|first5=Ulf|title=Synthesis and reactions of 1,1-diamino-2,2-dinitroethylene|url=http://linkinghub.elsevier.com/retrieve/pii/S0040402098006735|journal=Tetrahedron|volume=54|issue=38|pages=11525–11536|doi=10.1016/s0040-4020(98)00673-5}}</ref> |
||
Due to its small-scale production, the cost of FOX-7 is relatively high. However, the production is based on commercial starting material and the synthesis is uncomplicated.<ref>US Patent 6312538 - Chemical compound suitable for use as an explosive, intermediate and method for preparing the compound<</ref> The price is therefore predicted to fall as production scale increases. There is no current full scale use of FOX-7, but it is being tested at several military research centers. The need for less sensitive munitions is the most important driver for testing FOX-7. |
Due to its small-scale production, the cost of FOX-7 is relatively high. However, the production is based on commercial starting material and the synthesis is uncomplicated.<ref>US Patent 6312538 - Chemical compound suitable for use as an explosive, intermediate and method for preparing the compound<</ref> The price is therefore predicted to fall as production scale increases. There is no current full scale use of FOX-7, but it is being tested at several military research centers. The need for less sensitive munitions is the most important driver for testing FOX-7. |
||
Revision as of 20:54, 12 February 2018
| |
| Names | |
|---|---|
| IUPAC name
2,2-Dinitroethene-1,1-diamine
| |
| Other names
FOX-7
FOX7 | |
| Identifiers | |
3D model (JSmol)
|
|
| ECHA InfoCard | 100.130.630 |
CompTox Dashboard (EPA)
|
|
| |
| Properties | |
| C2H4N4O4 | |
| Molar mass | 148.08 |
| Density | 1.885 g cm−3 |
| Melting point | 238 °C (460 °F; 511 K) (decomposes) |
| Explosive data | |
| Detonation velocity | 8870 m/s at density 1.885 g cm−3 (estimated) 8335 m/s at density 1.756 g cm−3 (measured, small-scale testing) |
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa).
| |
FOX-7 or 1,1-diamino-2,2-dinitroethene (DADNE)[1] is an insensitive high explosive compound. It was first synthesized in 1998 by the Swedish National Defence Research Institute (FOA).[2]
FOX-7 is similar to the insensitive chemical compound TATB, which is a benzene ring compound with three amino and three nitro groups.[3] FOX-7 has a two-carbon backbone rather than a benzene ring, but the amino and nitro groups have similar effects in both cases according to published reports on sensitivity and chemical decay processes of FOX-7. FOX-7 is today produced by Eureco Bofors AB in Sweden.[4]
Its explosive properties appear extremely favorable; in addition to its insensitive properties, the detonation velocity of mixtures of 80% FOX-7 plus binders is as high as Composition B, and nearly pure FOX-7 based plastic bonded explosives are slightly superior to RDX. [5] FOX-7 has been calculated to have a detonation velocity of 8,870 m/s.[6]
Due to its small-scale production, the cost of FOX-7 is relatively high. However, the production is based on commercial starting material and the synthesis is uncomplicated.[7] The price is therefore predicted to fall as production scale increases. There is no current full scale use of FOX-7, but it is being tested at several military research centers. The need for less sensitive munitions is the most important driver for testing FOX-7.
References
- ^ US Patent 6340780 - Method of preparing salts of dinitromethane
- ^ Bemm, U.; Östmark, H. (1998) "1,1-Diamino-2,2-dinitroethylene: a Novel Energetic Material with Infinite Layers in Two Dimensions." Acta Cryst C54: 1–3. doi:10.1107/S0108270198007987
- ^ Hervé, Grégoire; Jacob, Guy; Latypov, Nikolaj (2005). "The reactivity of 1,1-diamino-2,2-dinitroethene (FOX-7)". Tetrahedron. 61 (28): 6743. doi:10.1016/j.tet.2005.05.010.
- ^ Anniyappan, M.; Talawar, M.B.; Gore, G.M.; Venugopalan, S.; Gandhe, B.R. (2006). "Synthesis, characterization and thermolysis of 1,1-diamino-2,2-dinitroethylene (FOX-7) and its salts". Journal of Hazardous Materials. 137 (2): 812–9. doi:10.1016/j.jhazmat.2006.03.034. PMID 16701943.
- ^ DETONATION AND SENSITIVITY PROPERTIES OF FOX-7 AND FORMULATIONS CONTAINING FOX-7, Karlsson et al., 2002, accessed Aug 25, 2005
- ^ Latypov, Nikolai V.; Bergman, Jan; Langlet, Abraham; Wellmar, Ulf; Bemm, Ulf. "Synthesis and reactions of 1,1-diamino-2,2-dinitroethylene". Tetrahedron. 54 (38): 11525–11536. doi:10.1016/s0040-4020(98)00673-5.
- ^ US Patent 6312538 - Chemical compound suitable for use as an explosive, intermediate and method for preparing the compound<
Further reading
- Sorescu, Dan C.; Boatz, Jerry A.; Thompson, Donald L. (2001). "Classical and Quantum-Mechanical Studies of Crystalline FOX-7 (1,1-Diamino-2,2-dinitroethylene)". The Journal of Physical Chemistry A. 105 (20): 5010. doi:10.1021/jp010289m.
- Evers, Jürgen; Klapötke, Thomas M.; Mayer, Peter; Oehlinger, Gilbert; Welch, Jan (2006). "Α- and β-FOX-7, Polymorphs of a High Energy Density Material, Studied by X-ray Single Crystal and Powder Investigations in the Temperature Range from 200 to 423 K". Inorganic Chemistry. 45 (13): 4996–5007. doi:10.1021/ic052150m. PMID 16780321.
