Iron(II) oxalate: Difference between revisions
m →top: Typo fixing, replaced: a inorganic compound → an inorganic compound |
Eudialytos (talk | contribs) natural occurrence |
||
| Line 56: | Line 56: | ||
When heated, it dehydrates and decomposes into a mixture of iron oxides and [[pyrophoric]] iron metal, with release of [[carbon dioxide]], [[carbon monoxide]], and water.<ref>{{cite journal |title= Thermal behaviour of iron(II) oxalate dihydrate in the atmosphere of its conversion gases |first1= Martin |last1=Hermanek |first2=Radek |last2=Zboril |first3=Miroslav |last3=Mashlan |first4=Libor |last4=Machala |first5=Oldrich |last5=Schneeweiss |display-authors= 3 |journal= J. Mater. Chem. |date= 2006 |volume= 16 |pages= 1273–1280}}</ref> |
When heated, it dehydrates and decomposes into a mixture of iron oxides and [[pyrophoric]] iron metal, with release of [[carbon dioxide]], [[carbon monoxide]], and water.<ref>{{cite journal |title= Thermal behaviour of iron(II) oxalate dihydrate in the atmosphere of its conversion gases |first1= Martin |last1=Hermanek |first2=Radek |last2=Zboril |first3=Miroslav |last3=Mashlan |first4=Libor |last4=Machala |first5=Oldrich |last5=Schneeweiss |display-authors= 3 |journal= J. Mater. Chem. |date= 2006 |volume= 16 |pages= 1273–1280}}</ref> |
||
==Natural occurrence== |
|||
Anhydrous iron(II) oxalate is as yet (2020) unknown among minerals. However, the dihydrate is known as humboldtine.<ref>https://www.mindat.org/min-1946.html</ref><ref name=IMA>https://www.ima-mineralogy.org/Minlist.htm</ref> A related, though much more complex mineral is [[stepanovite]], Na[Mg(H<sub>2</sub>O)<sub>6</sub>][Fe(C<sub>2</sub>O<sub>4</sub>)<sub>3</sub>]·3H<sub>2</sub>O - an example of trioxalatoferrate(II).<ref>https://www.mindat.org/min-3763.html</ref><ref name=IMA>https://www.ima-mineralogy.org/Minlist.htm</ref> |
|||
==See also== |
==See also== |
||
Revision as of 12:34, 15 November 2020

| |

| |
| Names | |
|---|---|
| IUPAC name
Iron(II) oxalate
| |
| Other names
Iron oxalate
Ferrous oxalate | |
| Identifiers | |
3D model (JSmol)
|
|
| ECHA InfoCard | 100.007.472 |
| EC Number |
|
PubChem CID
|
|
| UNII |
|
CompTox Dashboard (EPA)
|
|
| |
| |
| Properties | |
| FeC2O4 (anhydrous) FeC2O4 · 2 H2O (dihydrate) | |
| Molar mass | 143.86 g/mol (anhydrous) 179.89 g/mol (dihydrate) |
| Appearance | yellow powder |
| Odor | odorless |
| Density | 2.28 g/cm3 |
| Melting point | 190 °C (374 °F; 463 K) (anhydrous)[1] 150–160 °C (302–320 °F; 423–433 K) (dihydrate) decomposes |
| Boiling point | 365.1 °C (689.2 °F; 638.2 K) (anhydrous)[1] |
| dihydrate: 0.097 g/100ml (25 °C)[2] | |
| Hazards | |
| GHS labelling: | |
 [3] [3]
| |
| Warning | |
| H302, H312[3] | |
| P280[3] | |
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa).
| |
Ferrous oxalate, or iron(II) oxalate, is an inorganic compound with the formula FeC2O4 · x H2O where x is typically 2. These are orange compounds, poorly soluble in water.
Structure
The dihydrate FeC2O4 · 2 H2O is a coordination polymer, consisting of chains of oxalate-bridged ferrous centers, each with two aquo ligands.[4]
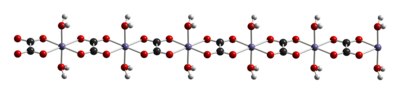
When heated, it dehydrates and decomposes into a mixture of iron oxides and pyrophoric iron metal, with release of carbon dioxide, carbon monoxide, and water.[5]
Natural occurrence
Anhydrous iron(II) oxalate is as yet (2020) unknown among minerals. However, the dihydrate is known as humboldtine.[6][7] A related, though much more complex mineral is stepanovite, Na[Mg(H2O)6][Fe(C2O4)3]·3H2O - an example of trioxalatoferrate(II).[8][7]
See also
A number of other iron oxalates are known
References
- ^ a b http://www.guidechem.com/cas-516/516-03-0.html
- ^ http://chemister.ru/Database/properties-en.php?dbid=1&id=2084
- ^ a b c Sigma-Aldrich Co., Iron(II) oxalate dihydrate. Retrieved on 2014-05-03.
- ^ Echigo, Takuya; Kimata, Mitsuyoshi (2008). "Single-crystal X-ray diffraction and spectroscopic studies on humboldtine and lindbergite: weak Jahn–Teller effect of Fe2+ ion". Phys. Chem. Minerals. 35: 467–475. doi:10.1007/s00269-008-0241-7.
- ^ Hermanek, Martin; Zboril, Radek; Mashlan, Miroslav; et al. (2006). "Thermal behaviour of iron(II) oxalate dihydrate in the atmosphere of its conversion gases". J. Mater. Chem. 16: 1273–1280.
- ^ https://www.mindat.org/min-1946.html
- ^ a b https://www.ima-mineralogy.org/Minlist.htm
- ^ https://www.mindat.org/min-3763.html
