From Wikipedia, the free encyclopedia
Chemical compound
Cyclotriol Other names ZK-136295; Cycloestriol; 14α,17α-Ethanoestriol; 14α,17α-Ethanoestra-1,3,5(10)-triene-3,16α,17β-triol; 14,21-Cyclo-19-norpregna-1,3,5(10)-triene-3,16α,17α-triol Routes of By mouth [1] Drug class Estrogen Bioavailability 40%[1] Elimination half-life 12.3 hours[1]
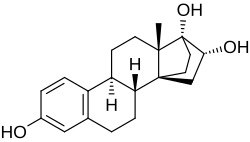
(8R ,9S ,13S ,14S ,16R ,17R )-13-Methyl-7,8,9,11,12,13,15,16-octahydro-14,17-ethanocyclopenta[a ]phenanthrene-3,16,17(6H )-triol
CAS Number PubChem CID ChemSpider UNII CompTox Dashboard (EPA ) Formula C 20 H 26 O 3 Molar mass −1 3D model (JSmol )
C[C@]12CC[C@H]3[C@H]([C@@]14CC[C@@]2([C@@H](C4)O)O)CCC5=C3C=CC(=C5)O
InChI=1S/C20H26O3/c1-18-7-6-15-14-4-3-13(21)10-12(14)2-5-16(15)19(18)8-9-20(18,23)17(22)11-19/h3-4,10,15-17,21-23H,2,5-9,11H2,1H3/t15-,16-,17-,18+,19+,20+/m1/s1
Key:PAMNOUDFJQSYMD-MUJBESKKSA-N
Cyclotriol (developmental code name ZK-136295 ; also known as 14α,17α-ethanoestriol ) is a synthetic estrogen which was studied in the 1990s and was never marketed.[2] [1] [3] [4] derivative of estriol with a bridge between the C14α and C17α positions.[2] [1] [3] [5] relative binding affinity of estradiol for the human ERα .[2] absolute bioavailability of 40% with high interindividual variability and an elimination half-life of 12.3 hours in pharmacokinetic studies in women.[1]
^ a b c d e f Baumann A, Fuhrmeister A, Brudny-Klöppel M, Draeger C, Bunte T, Kuhnz W (October 1996). "Comparative pharmacokinetics of two new steroidal estrogens and ethinylestradiol in postmenopausal women". Contraception . 54 (4): 235–242. doi :10.1016/S0010-7824(96)00194-1 . PMID 8922877 . ^ a b c Oettel M, Schillinger E (6 December 2012). Estrogens and Antiestrogens II: Pharmacology and Clinical Application of Estrogens and Antiestrogen ISBN 978-3-642-60107-1 ^ a b Lang R, Reimann R (1993). "Studies for a genotoxic potential of some endogenous and exogenous sex steroids. I. Communication: examination for the induction of gene mutations using the Ames Salmonella/microsome test and the HGPRT test in V79 cells". Environmental and Molecular Mutagenesis . 21 (3): 272–304. doi :10.1002/em.2850210311 . PMID 8462531 . S2CID 39049586 . ^ Reimann R, Kalweit S, Lang R (1996). "Studies for a genotoxic potential of some endogenous and exogenous sex steroids. II. Communication: examination for the induction of cytogenetic damage using the chromosomal aberration assay on human lymphocytes in vitro and the mouse bone marrow micronucleus test in vivo". Environmental and Molecular Mutagenesis . 28 (2): 133–144. doi :10.1002/(SICI)1098-2280(1996)28:2<133::AID-EM10>3.0.CO;2-G . PMID 8844995 . S2CID 10326219 . ^ Hundal BS, Dhillon VS, Sidhu IS (March 1997). "Genotoxic potential of estrogens". Mutation Research . 389 (2–3): 173–181. doi :10.1016/S1383-5718(96)00144-1 . PMID 9093381 .
ER Tooltip Estrogen receptor
Agonists
Steroidal: 2-Hydroxyestradiol 2-Hydroxyestrone 3-Methyl-19-methyleneandrosta-3,5-dien-17β-ol 3α-Androstanediol 3α,5α-Dihydrolevonorgestrel 3β,5α-Dihydrolevonorgestrel 3α-Hydroxytibolone 3β-Hydroxytibolone 3β-Androstanediol 4-Androstenediol 4-Androstenedione 4-Fluoroestradiol 4-Hydroxyestradiol 4-Hydroxyestrone 4-Methoxyestradiol 4-Methoxyestrone 5-Androstenediol 7-Oxo-DHEA 7α-Hydroxy-DHEA 7α-Methylestradiol 7β-Hydroxyepiandrosterone 8,9-Dehydroestradiol 8,9-Dehydroestrone 8β-VE2 10β,17β-Dihydroxyestra-1,4-dien-3-one (DHED) 11β-Chloromethylestradiol 11β-Methoxyestradiol 15α-Hydroxyestradiol 16-Ketoestradiol 16-Ketoestrone 16α-Fluoroestradiol 16α-Hydroxy-DHEA 16α-Hydroxyestrone 16α-Iodoestradiol 16α-LE2 16β-Hydroxyestrone 16β,17α-Epiestriol (16β-hydroxy-17α-estradiol) 17α-Estradiol (alfatradiol )17α-Dihydroequilenin 17α-Dihydroequilin 17α-Epiestriol (16α-hydroxy-17α-estradiol) 17α-Ethynyl-3α-androstanediol 17α-Ethynyl-3β-androstanediol 17β-Dihydroequilenin 17β-Dihydroequilin 17β-Methyl-17α-dihydroequilenin Abiraterone Abiraterone acetate Alestramustine Almestrone Anabolic steroids (e.g., testosterone and esters , methyltestosterone , metandienone (methandrostenolone) , nandrolone and esters , many others; via estrogenic metabolites)Atrimustine Bolandiol Bolandiol dipropionate Butolame Clomestrone Cloxestradiol
Conjugated estriol Conjugated estrogens Cyclodiol Cyclotriol DHEA DHEA-S ent -EstradiolEpiestriol (16β-epiestriol, 16β-hydroxy-17β-estradiol) Epimestrol Equilenin Equilin ERA-63 (ORG-37663) Esterified estrogens Estetrol Estradiol
Estramustine Estramustine phosphate Estrapronicate Estrazinol Estriol
Estrofurate Estrogenic substances Estromustine Estrone
Etamestrol (eptamestrol) Ethinylandrostenediol
Ethinylestradiol
Ethinylestriol Ethylestradiol Etynodiol Etynodiol diacetate Hexolame Hippulin Hydroxyestrone diacetate Lynestrenol Lynestrenol phenylpropionate Mestranol Methylestradiol Moxestrol Mytatrienediol Nilestriol Norethisterone Noretynodrel Orestrate Pentolame Prodiame Prolame Promestriene RU-16117 Quinestradol Quinestrol Tibolone Xenoestrogens: Anise -related (e.g., anethole , anol , dianethole , dianol , photoanethole )Chalconoids (e.g., isoliquiritigenin , phloretin , phlorizin (phloridzin) , wedelolactone )Coumestans (e.g., coumestrol , psoralidin )Flavonoids (incl. 7,8-DHF , 8-prenylnaringenin , apigenin , baicalein , baicalin , biochanin A , calycosin , catechin , daidzein , daidzin , ECG , EGCG , epicatechin , equol , formononetin , glabrene , glabridin , genistein , genistin , glycitein , kaempferol , liquiritigenin , mirificin , myricetin , naringenin , penduletin , pinocembrin , prunetin , puerarin , quercetin , tectoridin , tectorigenin )Lavender oil Lignans (e.g., enterodiol , enterolactone , nyasol (cis -hinokiresinol) )Metalloestrogens (e.g., cadmium )Pesticides (e.g., alternariol , dieldrin , endosulfan , fenarimol , HPTE , methiocarb , methoxychlor , triclocarban , triclosan )Phytosteroids (e.g., digitoxin (digitalis ), diosgenin , guggulsterone )Phytosterols (e.g., β-sitosterol , campesterol , stigmasterol )Resorcylic acid lactones (e.g., zearalanone , α-zearalenol , β-zearalenol , zearalenone , zeranol (α-zearalanol) , taleranol (teranol, β-zearalanol) )Steroid -like (e.g., deoxymiroestrol , miroestrol )Stilbenoids (e.g., resveratrol , rhaponticin )Synthetic xenoestrogens (e.g., alkylphenols , bisphenols (e.g., BPA , BPF , BPS ), DDT , parabens , PBBs , PHBA , phthalates , PCBs )Others (e.g., agnuside , rotundifuran ) MixedSERMs Tooltip Selective estrogen receptor modulators ) Antagonists
Coregulator-binding modulators: ERX-11
GPER Tooltip G protein-coupled estrogen receptor
Agonists Antagonists Unknown