Breast augmentation

Breast augmentation denotes surgical and non-surgical mammoplasty procedures to alter and correct the size, form, and feel of a woman’s breasts. The surgical approach features global augmentation with a breast implant prosthesis and corrections with transplanted skin flaps. The non-surgical approach features breast augmentation and corrections by contouring the recipient site with grafts of autologous fat tissue. Each approach corrects post–mastectomy defects in breast reconstruction; congenital chest wall deformities; and effects the aesthetic enhancement of the bust. In surgical practice, the breast tissue expander implant is a temporary breast-implant device used in staged breast reconstruction. In non-surgical practice, some breast surgery fat graft corrections feature pre-procedure external tissue expansion of the recipient site.
Surgical breast augmentation
Breast implants
To recreate and enhance the size, form, and feel of a woman’s breasts, breast implant devices come in three types: saline implants filled with sterile saline solution, silicone implants filled with viscous silicone gel, and alternative-composition implants, filled with miscellaneous fillers — soy oil, polypropylene string, et cetera — that are no longer manufactured.

Saline implants
Breast implants filled with saline solution (salt water) of biological concentration were first manufactured in 1964, in France, and introduced for medical use in 1964, the surgico–technical goals were emplacement via shorter incisions.[1] Unlike the predecessor models, modern saline breast have shells made of room-temperature vulcanized (RTV) elastomer silicone. During the surgery, the empty implants are filled with saline solution, after emplacement to the implant pocket. Because the implants are inserted empty, the resultant surgical-incision scar will be smaller than the scar produced by the long-incision IMF (inframammary fold) approach used for inserting the comparatively voluminous, pre-filled silicone gel breast implants.[2] Although the silicone breast implant has superior aesthetic characteristics, the saline implant, if correctly emplaced, produces good-to-excellent results might results, despite a tendency to cause readily noticeable cosmetic problems, such as rippling and wrinkling. In patients with more breast tissue, for whom submuscular implant placement is the recommended surgical technique, saline breast implants can afford an aesthetic appearance much like that afforded by the silicone-gel breast implant.

Silicone gel implants
Silicone gel breast implants are described in five implant device generations, defined by the manufacturing techniques meant to improve its physical and aesthetic realism, as if a natural breast. The silicone breast implant was invented in 1961 by the American plastic surgeons Thomas Cronin and Frank Gerow, and the Dow Corning Corporation; and the first augmentation mammoplasty was performed in 1962, using the silicone gel Cronin–Gerow Implant, prosthesis model 1963.
First generation (1960s) — The outcomes of mammoplastic praxis and aesthetic surgery were much improved by the introduction of the Cronin–Gerow Implant (prosthesis model 1963), because it was an anatomically shaped (contoured) breast implant device — a tear-drop silicone rubber envelope filled with viscous silicone-gel — that better resembled a natural breast. To reduce the rotation of the emplaced implant device, the Cronin–Gerow Implant featured a fastener-patch, of Dacron (Polyethylene terephthalate), affixed to the back of the device shell.[3]
Second generation (1970s) — The notable design and technological improvements to the breast implant included greater physical verisimilitude, an anti-inflammatory coating, and a double-cavity construction. The softer, more life-like breast implants, because of the thinner device shells and thinner (low-cohesion) silicone-gel filler, but had greater incidences of shell rupture and filler leakage through an intact implant-device shell, and of complications (e.g. capsular contracture). In the early 1990s, faulty, Second-generation silicone-gel breast implants were the subjects of U.S. government class action lawsuits against the Dow Corning Corporation, and other breast implant device manufacturers.
The polyurethane foam coating for the implant shell reduced incidences of capsular contracture by causing an inflammatory reaction that impeded the formation of a scar capsule — fibrous collagen tissue — around the breast implant. Yet coated models were temporarily disallowed until the U.S. Food and Drug Administration (FDA) determined whether or not there existed a health risk from the chemical 2,4-toluenediamine (TDA), a carcinogenic by-product of the chemical breakdown of the implant’s polyurethane foam coating; after investigating, the FDA re-authorized their plastic surgery use in the U.S.[4][5]
The double lumen design — a silicone implant within a saline implant — meant to provide the physical verisimilitude and aesthetic benefits of silicone-gel (inner lumen) enclosed in saline solution (outer lumen). The volume (size) of the double-cavity breast implant device was post-operatively adjustable, yet the more complex design had a device-failure rate greater than that of single-lumen implant devices. A modern version of the double lumen design is the “Becker Expandable” (1984) implant, which is used primarily as a recipient site tissue expander in breast reconstruction.
Third generation and Fourth generation (1980s) — Introduced in mid-decade, elastomer-coated device shells, increased-cohesion gel filler, and model variety, were the technological advances in manufacturing silicone breast implants. The elastomer silicone coating of the implant device shell, and an increased-cohesion (thicker) filler gel, decreased the incidence of filler leakage. The manufacturers then introduced breast implants in anatomic (natural) and shaped (round, contoured) design varieties, available with either a smooth or a textured surface to control the movement of the emplaced implant device.
Fifth generation (1990s) — Introduced in mid-decade, the silicone breast implant is of semi-solid gel that mostly eliminates filler leakage (silicone migration), thus has reported low incidence rates of capsular contracture and of device-shell rupture.[6][7][8]
Implants and breast feeding
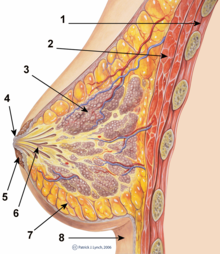
Legend:
1. Chest wall
2. Pectoralis muscles
3. Lobules
4. Nipple
5. Areola
6. Duct
7. Fatty tissue
8. Skin
Women with breast implants are able to breast-feed their infants, yet breast implant devices can cause functional breast-feeding difficulties, because some mammoplasty procedures — especially periareolar incisions and subglandular emplacement — are associated with greater incidences of impeded breast-feeding. If she wishes to ensure maximal breast-feeding functionality post-surgery, the child-bearing-age woman seeking breast implants discusses with her plastic surgeon the best implantation procedure that will least damage her milk ducts and nipple nerves to allow nursing her infant.[9][10][11]

Breast-feeding an infant.
The principal infant-health concerns are leakage of breast-implant filler to the breast milk, and if the filler is dangerous to the nursing infant. Breast implant device fillers are biologically inert — saline filler is salt water, and silicone filler is indigestible — each substance is chemically neutral and environmentally common.[12][13] In the early 1990s, at the beginning of the silicone breast-implant sickness occurrences, small-scale, non-random studies indicated possible breast-feeding complications from silicone implants; yet no study reported device–disease causality.[13]
Functional breast-feeding difficulies arise if the surgeon cut the milk ducts or the major nerves, or if the milk glands were otherwise damaged. Milk duct and nerve damage are more common to the periareolar incision implantation procedure, which cuts tissue near the nipple, whereas other incisions — inframammary, TABA, transaxillary, TUBA — avoid the nipple tissue.[11] To maximally preserve breast-feeding functionality, the periareolar incisions can be effected to reduce damage to the milk ducts and major nerves. The milk glands are most affected by subglandular (over-the-muscle) implants, and by large-sized breast implants, which pinch the gland and impede breast milk flow. Submuscular implants and small-sized implants cause fewer breast-function problems; nonetheless, women have successfully breast-fed after undergoing augmentation mammoplasty with periareolar incisions and subglandular implant placement.[11]
Implants and mammography

The presence of radiologically opaque breast implants might interfere with the radiographic sensitivity of the mammograph. In which case, an Eklund view mammogram is required, wherein the breast implant is manually displaced against the chest wall and the breast is pulled forward, so that the mammograph can visualize the internal tissues, nonetheless, approximately one-third (1/3) of the breast tissue remains inadequately visualized, resulting in an increased incidence of false-negative mammograms.[14] Breast cancer studies of women with implants reported no significant differences in “stage-of-disease” at the time of diagnosis; the prognoses are similar in both groups, with augmented patients at a lower risk for subsequent cancer recurrence or death.[15][16] Conversely, the use of implants for breast reconstruction after breast cancer mastectomy appears to have no negative effect upon the incidence of cancer-related death.[17]
That patients with breast implants are more often diagnosed with palpable – but not larger – tumors, indicates that equal-sized tumors might be more readily palpated in augmented patients, which might compensate for the impaired mammogram images.[18] The palpability is consequent to breast tissue thinning by compression, innately smaller breasts a priori, and that the implant serves as a radio-opaque base against which a cancerous tumor can be differentiated.[19] The implant device has no clinical bearing upon lumpectomy breast conservation surgery for patients who developed breast cancer post-implantation, and it does not interfere with external beam radiation treatments (XRT); post-treatment incidence of breast-tissue fibrosis is common, and thus an increased rate of capsular contracture.[20]
Surgical procedures
Indications

An augmenation mammoplasty for emplacing breast implants has three purposes:
- primary reconstruction — to replace breast tissues damaged by trauma (blunt, penetrating), disease (breast cancer), or failed anatomic development (tuberous breast deformity).
- revision and reconstruction — to revise (correct) the outcome of a previous breast reconstruction surgery.
- primary augmentation — to aesthetically augment the size, form, and feel of the breasts.
The operating room (OR) time of post–mastectomy breast reconstruction, and of breast augmentation surgery is determined by the procedure employed, the type of incisions, the breast implant (type and materials), and the pectoral locale of the implant pocket.
Incision types
Breast implant device emplacement is performed with five types of surgical incisions:
- Inframammary — an incision made below the breast, in the infra-mammary fold (IMF), which affords maximal access for precise dissection and emplacement of the breast implant devices. It is the preferred surgical technique for emplacing silicone-gel implants, because of the longer incisions required; yet, IMF implantation can produce thicker, slightly more visible surgical scars.
- Periareolar — an incision made along the areolar periphery (border), which provides an optimal approach when adjustments to the IMF position are required, or when a mastopexy (breast lift) is included to the primary mammoplasty procedure. In the periareolar emplacement method, the incision is around the medial-half (inferior half) of the areola’s circumference. Silicone-gel implants can be difficult to emplace with this incision, because of the short, five-centimetre length (~ 5.0 cm.) of the required access-incision. Aesthetically, because the scars are at the areola’s border, they usually are less visible than the IMF-incision scars of women with light-pigment areolae. Furthermore, periareolar implantation produces a greater incidence of capsular contracture, severs the milk ducts and the nerves to the nipple, thus causes the most post-operative functional problems, e.g. impeded breast feeding.
- Transaxillary — an incision made to the axilla (armpit), from which the dissection tunnels medially, thus allows emplacing the implants without producing visible scars upon the breast proper; yet is likelier to produce inferior asymmetry of the implant-device position. Therefore, surgical revision of transaxillary emplaced breast implants usually requires either an IMF incision or a periareolar incision. Transaxillary emplacement can be performed bluntly or with an endoscope (illuminated video microcamera).
- Transumbilical — a trans-umbilical breast augmentation (TUBA) is a less common implant-device insertion technique wherein the incision is at the navel, and the dissection tunnels superiorly. This surgical approach enables emplacing the breast implants without producing visible scars upon the breast; but it makes appropriate dissection and device-emplacement more technically difficult. A TUBA procedure is performed bluntly — without the endoscope’s visual assistance — and is not appropriate for emplacing (pre-filled) silicone-gel implants, because of the great potential for damaging the elastomer silicone shell of the breast-implant device during its manual insertion through the short — two-centimetre (~2.0 cm.) — incision at the navel, and because pre-filled silicone-gel implants are incompressible, and cannot be inserted through so small an incision.[21]
- Transabdominal — as in the TUBA procedure, in the transabdominoplasty breast augmentation (TABA), the breast implants are tunneled superiorly from the abdominal incision into bluntly dissected implant pockets, whilst the patient simultaneously undergoes an abdominoplasty.[22]
Implant pocket placement
Cross-section of a subglandular breast prosthesis emplacement (1), and of a submuscular breast prosthesis emplacement (2).
The surgical emplacement of breast implant devices to the implant pocket is described in relation to the pectoralis major muscle.
- Subglandular — implantation between the breast tissue and the pectoralis major muscle, in the retromammary space. This position closely approximates the plane of normal breast tissue, and affords the most aesthetic results; but, in thin soft-tissue patients, the subglandular position is likelier to show the ripples and wrinkles of the underlying breast implant; moreover, the capsular-contracture rate is slightly greater.
- Subfascial — the breast implant device is emplaced underneath the fascia of the pectoralis major muscle; a variant of the subglandular position.[23] The technical benefits of the subfascial breast-implant pocket technique are debated; proponent surgeons report that the layer of fascial tissue provides greater implant coverage and better sustains its position. [24]
- Subpectoral (dual plane) — the breast implant device is emplaced beneath the pectoralis major muscle, after releasing the inferior muscular attachments. Resultantly, the implant device is partially beneath the pectoralis major muscle in the upper pole, while the implant’s lower half is in the subglandular plane. This implantation technique achieves maximal upper-implant coverage, whilst allowing the expansion of the lower pole; however, animation (movement) of the breast implants in the subpectoral plane can be excessive for some patients. [25]
- Submuscular — the implant device is emplaced below the pectoralis major muscle, without releasing the inferior origin of the muscle. Total muscular coverage of the breast implant device can be achieved by releasing the lateral muscles of the chest wall — either the serratus muscle or the pectoralis minor muscle, or both — and suturing it, or them, to the pectoralis major muscle. In breast reconstruction surgery, this emplacement technique effects maximal coverage of the breast implant devices.
Post-surgical recovery
The surgical scars of a breast augmentation mammoplasty last about six weeks and fade within months. Depending upon the daily physical activity each woman might require, breast augmentation surgery patients usually resume their normal lives about one week post-operative. Women whose breast implants were emplaced beneath the chest muscles (submuscular placement) usually have a longer post–operative convalescence, and experience slightly more pain, because of the incisions done to the chest muscles during the augmenation mammoplasty. The patient usually does not exercise or engage in strenuous physical activities for about six weeks. During the initial recovery, the patient is encouraged to regularly exercise (flex and move) her arm to alleviate pain and discomfort; also available are analgesic medication catheters for alleviating pain.[26][27]
Complications
The surgical emplacement of breast implant devices — whether for breast reconstruction or for aesthetic purpose — presents the same health risks common to surgery, such as adverse reaction to anesthesia, hematoma (post–operative bleeding), seroma (fluid accumulation), site breakdown (infection); and especially breast pain, altered sensation, impeded breast-feeding, visible wrinkling, asymmetry, thinning of the breast tissue, and symmastia “bread loafing”, the disruption of the natural plane between the breasts. Specific treatments for the complications of indwelling breast implants — capsular contracture and capsular rupture — are periodic MRI monitoring and physical examinations. Furthermore, complications and re-operations related to the implantation surgery, and to tissue expanders (implant place-holders during surgery) can cause unfavorable scarring in about 6–7 per cent of patients.[28][29]
Repair and revision surgeries
When the patient considers the outcome of an implantation mammoplasty unsatisfactory, or when techhnical or medical complications occur, or because of the breast implants’ limited product life, it is likely that women might have to replace their breast implants. The common re-operation (replacement) indications include major and minor complications, capsular contracture, shell rupture, and device deflation.[30] Re-operation rates were greater for breast reconstruction patients, because of the post-mastectomy changes in the soft-tissue envelope and to the anatomical breast borders, especially in patients who received adjuvant XRT (external radiation therapy).[30] Moreover, besides the reconstruction mammoplasty, breast cancer patients usually undergo surgeries of the nipple-areola complex (NAC), and symmetry procedures upon the opposite breast. Carefully matching of the patient’s soft-tissue characteristics to the type and size of the breast implants reduces having to re-operate. With appropriate implant device selection and proper implantation, the re-operation rate was 3.0 per cent at the 7-year-mark, compared with the re-operation rate of 20 per cent at the 3-year-mark, as reported by the U.S. Food and Drug Administration.[31][32]
Rupture

The principal breast-implant device rupture-rate factors are its age and design;[33] the suspected rupture mechanisms are: damage during implantation, damage during (other) surgical procedures, the chemical degradation of the implant’s shell, blunt chest trauma, penetrating chest trauma, and, infrequently, the mechanical pressure common to traditional mammographic breast examination.[30] Although implant devices can remain intact in a woman’s body for decades, they are a limited-life (years-long) Class III medical-device product.
When saline breast implants rupture, they usually quickly deflate, and thus are readily removed. Studies of women with saline implants reported 3-year-mark rupture-deflation rates of 3–5 per cent, and 10-year-mark rupture-deflation rates of 7–10 per cent.[34] When a silicone-gel breast implant device ruptures it usually does not deflate, yet the silicone-gel filler can leak from the device to the implant pocket, wherein rests the breast implant proper. An intracapsular rupture (in-capsule leak) can progress to extracapsular rupture (out-of-capsule leak); each condition generally indicates surgical removal of the breast implant device. Although extracapsular silicone can migrate from the chest to elsewhere in the patient’s body, most such clinical complications were limited to the breast and axillae areas, manifested as granulomas (inflammatory nodules) and axillary lymphadenopathy (enlarged lymph glands in the armpit area). [35] [36][37]
From the long-term MRI data for single-lumen breast implants, the European literature about silicone-gel breast implants designed in the 1970s (Second generation), reported silent device-rupture rates of 8–15 per cent at the 10-year-mark since implantation (15–30 per cent of patients).[38][39][40] In 2009, a branch study of the U.S. FDA’s core clinical trials for primary breast-augmentation surgery patients, reported device-rupture rates of 11 per cent at the 6-year-mark.[41] The first series of MRI evaluations of the silicone breast implants with thick filler gel had a device-rupture rate of 1.0 per cent, or less, at the median 6-year device-age.[42]
Capsular contracture

The human body’s immune response to surgically installed foreign objects — breast implants, cardiac pacemakers, orthopedic prostheses — is to cover them with scar tissue capsules of tightly woven collagen fibers; bodily integrity by isolation and toleration. Capsular contracture occurs when the collagen-fiber capsule thickens and compresses the breast implant, and is a painful complication that might distort the implant device or the breast, or both. The cause of capsular contracture is unknown, but the common incidence factors include bacterial contamination, device-shell rupture, filler leakage, and hematoma.
The device-implantation surgical procedures that have reduced the incidence of capsular contracture include submuscular implant placement, using textured-surface implant devices (polyurethane-coated);[18][43][44] limited handling of the implants, limited contact with the recipient site skin before emplacement, and irrigation with triple-antibiotic solutions.[45][46]
The correction of capsular contracture might require the surgical removal (release) of the collagen-fiber capsule, or the removal, and possible replacement, of the breast implant. Closed capsulotomy (disrupting the capsule via external manipulation), once was a common maneuver for treating hard capsules, but now is a discouraged technique, because it can rupture the breast implant. Non-surgical treatments for collagen-fiber capsules include massage, external ultrasonic therapy, leukotriene pathway inhibitors (e.g. Accolate, Singulair), and pulsed electromagnetic field therapy (PEMFT).[47][48][49][50]
Platinum toxicity
The metallic element platinum is a catalyst — a chemical that accelerates a reaction between two other chemicals, without, itself, forming part of the new compound chemical — thus transforming silicone oil into silicone gel for the manufacturing of the elastomer silicone shell of the breast implant device, and other medical-silicone devices.[51] The literature indicates that small amounts of platinum leak from such types of silicone breast-implant, therefore, platinum is present in the surrounding pectoral tissue. The rare pathogenic consequence is platinum accumulation in the bone marrow, from where blood cells might possibly deliver it to nerve endings, causing nervous system disorders such as involuntary tics, blindness, and deafness.[51] In 2002, the U.S. Food and Drug Administration reviewed the pertinent studies of the human biological effects of breast-implant platinum, and reported little causal evidence of platinum toxicity consequent to the platinum present in breast implant patients.[52]
Systemic sickness
Since the 1990s, comprehensive reviews of the studies for device–disease causal association between silicone-gel breast implants and systemic disease, reported no causal link between the silicone breast implants and subsequent systemic and auto-immune diseases.[53][54][55][56] Nonetheless, in that decade, thousands of women claimed breast-implant-caused sickness manifested as neurological and rheumatological health problems.[57] A Danish national health registry’s breast-implant study reported that women with implants did not risk a greater incidence–diagnosis of auto-immune disease, when compared to women of the same age group in the general populace; that the incidence of musculoskeletal disease was lower among women with breast implants, than among women who had undergone other types of cosmetic surgery, and than among women of the same age group in the general populace.[58][59] Follow-up longitudinal studies of these breast implant patients confirmed the previously reported findings on the matter.[60]
Other studies established that women who underwent augmentation mammoplasty — or any type of plastic surgery — tended to be healthier and wealthier than the general populace, before and after surgery. That plastic surgery patients had a decreased standardized mortality ratio, than did breast implant patients and patients for other plastic surgeries; yet had an increased risk of death by lung cancer than other plastic surgery patients. Because only one study controlled the factor of tobacco smoking; the data were insufficient to establish verifiable statistical differences between smoker and non-smoker that might contribute to the greater lung cancer death-rate of women with breast implants.[61][62] In 2006, a long-term follow-up study of some 25,000 Canadian women with breast implants, reported that the “findings suggest that breast implants do not directly increase mortality in women.”[63]
The study, Silicone gel Breast Implant Rupture, Extracapsular Silicone, and Health Status in a Population of Women (2001) reported an increased incidence of fibromyalgia among women who suffered extracapsular silicone-gel leakage, than among women whose breast implant devices neither ruptured nor leaked.[64] The study later was criticized as methodologically flawed, and later studies failed to establish such a causal device–disease association. After investigating, the U.S. FDA reported that “the weight of the epidemiological evidence published in the literature does not support an association between fibromyalgia and breast implants.”[65][66] Nonetheless — excluding the possibility that a small group of breast implant patients might sicken through (as yet) unknown disease mechanisms — the international medical consensus is that silicone-gel breast implant devices neither cause nor aggravate systemic and auto-immune diseases.[66]
Non-surgical breast augmentation
Background
Liposuction
The technique of liposuction (lipoplasty) was conceived by the Italian plastic surgeons Arpat Fischer and Giorgio Fischer in 1974, and was put into medical practice in 1975.[67] Autologous adipocyte fat was harvested by means of 5-mm incisions, and an electrically and pneumatically powered instrument that rotated and alternated in its aspiration of the fat through a cannula. Meanwhile, through a separate incision to the fat-tissue harvest site, saline solution was injected to dilute the fat and facilitate its aspiration.[68]
In 1977, Fisher and Fischer reviewed 245 cases with the “planotome” instrument for treating cellulite in the lateral trochanteric (hip-thigh) areas. There was a 4.9 per cent incidence of seromas, despite incision-wound suction catheters and compression dressings; 2.0 per cent of the cases presented pseudo-cyst formation that required removal of the capsule (cyst) through a wider incision (+ 5 mm) and the use of the panotome.[69][70]
The advent of liposuction technology facilitated medical applications of the liposuction-harvested fat tissue as autologous filler for injection to correct bodily defects, and for breast augmentation. Dr. Melvin Bircoll introduced the practice of contouring the breast and for correcting bodily defects with autologous fat grafts harvested by liposuction; and he presented the fat-injection method used for emplacing the fat grafts.[71][72] The surgeon E. Krulig emplaced fat grafts with a syringe and needle (lipo-injection), and later used a disposable fat trap to facilitate the collection and to ensure the sterility of the harvested adipocyte tissue.[73][74]
Lipo-injector gun
To emplace the autologous fat tissue grafts, Drs. J. Newman and J. Levin designed a lipo-injector gun with a gear-driven plunger that allowed the even injection of autologous fat tissue to the desired recipient sites. The control afforded by the injector gun assisted the plastic surgeon in controlling excessive pressure to the fat in the barrel of the syringe, thus avoiding over-filling the recipient site.[75] The later-design lipo-injector gun featured a ratchet-gear operation that that afforded the surgeon great control in accurately emplacing autologous fat to the recipient site; a trigger action injected 0.1 cc of filler.[76] Since 1989, most non-surgical, fat-graft augmentation of the breast features the emplacement of adipocyte fat outside the breast parenchyma — up to 300 ml of fat, in three equal measures (aliquots), is emplaced to the subpectoral space and to the intrapectoral muscle space of the pectoralis major muscle, and the submammary space, to achieve a breast outcome of natural appearance and contour.[77]
Autologous fat grafting
Autologous fat grafting to the breast is applied to the correction of breast asymmetry, the correction of breast deformities, breast reconstruction (as adjunct and primary technique), for the improvement of soft-tissue coverage of breast implants, and for the aesthetic enhancement of the bust. The careful harvesting and centrifugal refinement of the mature adipocyte tissue — to be injected in small aliquots — allows the transplanted fat tissue to remain viable in the breast and provide it the structure and contour that cannot be achieved solely with breast implants or with corrective surgery. As in all breast procedures, the grafted fat tissue can suffer necrosis, and concomitant calcifications and cysts, and palpable lumps; the cause of calcification is unknown, but the post-procedure tissue changes resemble those resulting from other breast procedures. The research indicates the efficacy of fat grafting for breast reconstruction the treatment of radiation damage to the chest, the incidental reduction of capsular contracture, and the improved soft-tissue coverage of breast implants.[78][79][80][81][82][83]
In Fat Grafting to the Breast Revisited: Safety and Efficacy (2007), Sydney Coleman and Alessia Saboeiro reported successful transferences of fat to the breast, and proposed fat grafting as a non-surgical alternative to the usual surgical procedures for breast augmentation, breast defect correction, and breast reconstruction. In a 17-patient group, 2 patients had breast cancer, diagnosed by mammogram, one at 12-months post-procedure, and the other at 92-months, after the fat transfer to the breast. In contemporary praxis, beyond the breast proper, fat grafts are injected to the pectoralis major muscle, and to the postpectoral space and to the prepectoral space, before and behind the muscle. In post-mastectomy breast reconstruction, the grafted fat is used to create a breast mound, by augmenting the extant breast tissues.[84]
To the group of 17 patients, structural fat grafting was performed to either one or both breasts; the age range of the women was 25–55 years, the mean age was 38.2 years. The indications included micromastia (10 patients); explantation deformity (one patient); post-augmentation deformity with breast implants (two patients); tuberous breast deformity (one patient); Poland’s syndrome (one patient); and post-mastectomy reconstruction deformity (two patients). The pre-proceduremammograms were negative for malignant neoplasms. The types of anaesthesia applied were general (two patients) and epidural analgesia plus sedation, with local infiltration and intercostal nerve blocks (15 patients). The autologous adipocyte tissue was grafted in one-to-three stages; the average tissue graft volume was 278.6 cc of fat per operation per breast. Post-procedure, the patient was instructed to regard any lump in the breasts as unrelated to the fat grafts, until after a complete medical workup of the breast lump had been performed.[85]
Techniques
Fat harvesting and contouring
The centrifugal refinement of the liposuction-harvested adipocyte tissues removes blood products and free lipids to produce autologous breast filler. The injectable filler-fat is obtained by centrifuging (spinning) the fat-filled syringes for sufficient time to allow the serum, blood, and oil (liquid fat) components to collect, by density, apart from the refined, injection-quality fat.[86] To refine the fat for facial injection quality, the fat-filled syringes are centrifuged for 1.0 minute at 2,000 RPM, which separates the unnecessary solution, leaving refined filler fat.[87] Moreover, centrifugation at 10,000 RPM for 10 minutes produces a “collagen graft”; the histologic composition of which is cell residues, collagen fibres, and 5.0 per cent intact fat cells. Furthermore, because the patient’s body naturally absorbs some of the fat grafts, the breasts maintain their contours and volumes for 18–24 months.[88][89]
In the Coleman–Saboeiro study, the autologous fat was harvested by liposuction, using a 10-ml syringe attached to a two-hole Coleman harvesting cannula; after centrifugation, the refined breast-filler fat was transferred to 3-ml syringes. Blunt infiltration cannulas were used to emplace the fat through 2-mm incisions; the blunt cannula injection method allowed greater dispersion of small aliquots (equal measures) of fat, and reduced the possibility of intravascular fat injection; no sharp needles are used for fat-graft injection to the breasts. The 2-mm incisions were positioned to allow the infiltration (emplacement) of fat grafts from at least two directions; a 0.2 ml fat volume was emplaced with each withdrawal of the cannula.[90]
The breasts were contoured by layering the fat grafts into different levels within the breast, until achieving the desired breast form. The fat-graft injection technique allows the plastic surgeon precise control in accurately contoruing the breast — from the chest wall to the breast-skin envelope — with subcutaneous fat grafts to the superficial planes of the breast. This greater degree of breast sculpting is unlike the global augmentation realised with a breast implant emplaced below the breast or below the pectoralis major muscle, respectively expanding the retromammary space and the retropectoral space. The greatest proportion of the grafted fat usually is infiltrated to the pectoralis major muscle, then to the retropectoral space, and to the prepectoral space, (before and behind the pectoralis major muscle). Moreover, although fat grafting to the breast parenchyma usually is minimal, it is performed to increase the degree of projection of the bust.[85]
Fat-graft injection
The biologic survival of autologous fat tissue depends upon the correct handling of the fat graft, of its careful washing (refinement) to remove extraneous blood cells, and of the controlled, blunt-cannula injection (emplacement) of the refined fat tissue grafts to an adequately vascularized recipient site. Because the body resorbs some of the injected fat grafts (volume loss), compensative over-filling aids in obtaining a satisfactory breast outcome for the patient; thus the transplantation of large-volume fat grafts greater than required, because only 25–50 per cent of the fat graft survives at 1-year post-transplantation.[91] Correct technique maximizes fat graft survival by minimizing cellular trauma during the liposuction harvesting and the centrifugal refinement, and by injecting the fat in small aliquots (equal measures), not clumps (too-large measures). Injecting minimal-volume aliquots with each pass of the cannula maximizes the surface area contact between the grafted fat tissue and the recipient breast-tissue, because proximity to a vascular system (blood supply) encourages histologic survival and minimizes the potential for fat necrosis.[92] Transplanted autologous fat tissue undergoes histologic changes like those undergone by a bone transplant; if the body accepts the fat-tissue graft, it is replaced with new fat tissue, if the graft dies it is replaced by fibrous tissue. New fat tissue is generated by the activity of a large, wandering histocyte-type cell, which ingests fat and then becomes a fat cell.[93] When the breast filler fat is injected to the breasts in clumps (too-large measures), fat cells emplaced too distant from blood vessels might die, which can lead to fat tissue necrosis, causing lumps, calcifications, and the eventual formation of liponecrotic cysts.
The operating room (OR) time required to harvest, refine, and emplace fat to the breasts is greater than the usual 2-hour OR time; the usual infiltration time was approximately 2-hours for the first 100 cc volume, and approximately 45 minutes for injecting each additional 100 cc volume of breast-filler fat. The technique for injecting fat grafts for breast augmentation allows the plastic surgeon great control in sculpting the breasts to the required contour, especially in the correction of tuberous breast deformity. In which case no fat graft is emplaced beneath the nipple-areola complex (NAC), and the skin envelope of the breast is selectively expanded (contoured) with fat emplaced subcutaneously, immediately beneath the skin. Such controlled contouring selectively increased the proportional volume of the breast in relation to the size of the areola-nipple complex, and thus created a breast of natural form and appearance; greater verisimilitude than is achieved solely with breast implants. The fat-corrected breast implant deformities were inadequate soft-tissue coverage of the implant(s) and capsular contracture, achieved with subcutaneous fat grafts that hid the implant-device edges and wrinkles, and decreased the palpability of the underlying breast implant. Furthermore, grafting autologous fat around the breast implant can result in softening the breast capsule.[94]
External tissue expansion
The successful outcome of fat graft breast augmentation requires a pre-expanded recipient site to create the breast-tissue matrix that will receive grafts of autologous adipocyte fat. The recipient site is expanded with an external vacuum tissue-expander applied upon each breast. The biological effect of negative pressure (vacuum) expansion upon soft tissues derives from the ability of soft tissues to grow when subjected to controlled, distractive, mechanical forces. (see distraction osteogenesis) The study Non-surgical Breast Enlargement using an External Soft Tissue Expansion System (2000) reported the technical effectiveness of recipient-site pre-expansion. In a single-group study, 17 healthy women (aged 18–40 y.o.) wore a brassiere-like vacuum system that applied a 20-mmHg vacuum (controlled, mechanical, distraction force) to each breast for 10–12 hours daily for 10-weeks. Pre- and post-procedure, the breast volume (size) was periodically measured; likewise, a magentic resonance image (MRI) of the breast-tissue architecture and water density was taken during the same phase of the patient’s menstrual cycle; of the 17-woman study group, 12 completed the study, and 5 withdrew, because of non-compliance with the clinical trial protocol.[95]
The breast volume (size) of all 17 women increased throughout the 10-week treatment period, the greatest increment was at week 10 (final treatment) — the average volume increase was 98+/–67 per cent over the initial breast-size measures. Incidences of partial recoil occurred at 1-week post-procedure, with no further, significant, breast volume decrease afterwards, nor at the follow-up treatment at 30-weeks post-procedure. The stable, long-term increase in breast size was 55 per cent (range 15–115%). The MRI visualizations of the breasts showed no edema, and confirmed the proportionate enlargement of the adipose and glandular components of the breast-tissue matrices. Furthermore, a statistically significant decrease in body weight occurred during the study, and self-esteem questionnaire scores improved from the initial-measure scores.[95]
Because external vacuum expansion of the recipient-site tissues permits injecting large-volume fat grafts (+ 300cc) to correct defects and enhance the bust, the histologic viability of the breast filler (adipocyte fat) and its volume must be monitored and maintained. The long-term, volume maintenance data reported in Breast Augmentation using Pre-expansion and Autologous Fat Transplantation: a Clinical Radiological Study (2010) indicate the technical effectiveness of external tissue expansion of the recipient site for a 25-patient study group, who had 46 breasts augmented with fat grafts. The indications included micromastia (under-development), explantation deformity (empty implant pocket), and congenital defects (tuberous breast deformity, Poland’s syndrome).[96] Pre-procedure, every patient used external vacuum expansion of the recipient-site tissues to create a breast-tissue matrix to be injected with autologous fat grafts of adipocyte tissue, refined via low G-force centrifugation. Pre- and post-procedure, the breast volumes were measured; the patients underwent pre-procedure and 6-month post-procedure MRI and 3-D volumetric imaging examinations. At 6-months post-procedure, each woman had a significant increase in breast volume, ranging 60–200 per cent, per the MRI (n=12) examinations. The size, form, and feel of the breasts was natural; post-procedure MRI examinations revealed no oil cysts or abnormality (neoplasm) in the fat-augmented breasts. Moreover, given the sensitive, biologic nature of breast tissue, periodic MRI and 3-D volumetric imaging examinations are required to monitor the breast-tissue viability and the maintenance of the large volume (+ 300cc) fat grafts.[96]
Indications
Non-surgical breast augmentation with injections of autologous fat grafts (adipocyte tissue) is indicated for patients requiring breast reconstruction, defect correction, and the æsthetic enhancement of the bust.
- breast reconstruction — post-mastectomy re-creation of the breast(s); trauma-damaged tissues (blunt, penetrating), disease (breast cancer), and explantation deformity (empty breast-implant socket).
- congenital defect correction — micromastia, tuberous breast deformity, Poland’s syndrome, et cetera.
- primary augmentation — the æsthetic enhancement (contouring) of the size, form, and feel of the breasts.
The operating room (OR) time of breast reconstruction, congenital defect correction, and primary breast augmentation procedures is determined by the indications to be treated.
Procedures
Post-mastectomy surgical reconstruction
Surgical post-mastectomy breast reconstruction requires general anaesthesia, cuts the chest muscles, produces new scars, and requires a long post-surgical recovery for the patient. The surgical emplacement of breast implant devices (saline or silicone) introduces a foreign object to the patient’s body (see capsular contracture). The TRAM flap (trans-abdominal mammoplasty) procedure reconstructs the breast using an autologous flap of muscle and tissues (harvested from either the abdomen or the back) and a breast implant. The DIEP flap (deep inferior epigastric perforators) procedure uses an autologous flap of abdominal skin and fat tissue — yet it does produce the most aesthetic outcome from surgical breast reconstruction.[97]
Post-mastectomy fat graft reconstruction
The reconstruction of the breast(s) with grafts of autologous fat is a non-surgical alternative to further surgery after a breast cancer surgery, be it a lumpectomy or a breast removal — simple (total) mastectomy, radical mastectomy, modified radical mastectomy, skin-sparing mastectomy, and subcutaneous (nipple-sparing) mastectomy. The breast is reconstructed by first applying external tissue expansion to the recipient-site tissues (adipose, glandular) to create a breast-tissue matrix that can be injected with autologous fat grafts (adipocyte tissue); the reconstructed breast has a natural form, look, and feel, and is generally sensate throughout and in the nipple-areola complex (NAC).[97] The reconstruction of breasts with fat grafts requires a 3-month treatment period — begun after 3–5 weeks of external vacuum expansion of the recipient-site tissues. The autologous breast-filler fat is harvested by liposuction from the patient’s body (buttocks, thighs, abdomen), is refined by syringe centrifuge, and then is injected (grafted) to the breast-tissue matrices (recipient sites), where the fat will thrive. The non-surgical breast reconstruction begins at the concluding steps of the breast cancer surgery, wherein the oncological surgeon is joined by the reconstructive plastic surgeon, who immediately begins harvesting, refining, and injecting fat grafts to the post-mastectomy recipient site. After that initial post-mastectomy fat grafting in the operating room, the patient leaves hospital with a slight breast mound that will become the foundation tissue matrix for the breast reconstruction.
After 3–5 weeks of continual external vacuum expansion of the breast mound (recipient site) — to promote the histologic regeneration of the extant tissues (fat, glandular) via increased blood circulation to the mastectomy scar (suture site) — the patient undergoes the first fat-grafting session for reconstructing her breasts. The external vacuum expansion of the breast mound created an adequate, vascularised, breast-tissue matrix to which the autologous fat is injected; and, per the patient, such reconstruction affords almost-normal sensation throughout the breast and the nipple-areola complex. Patient recovery from non-surgical fat graft breast reconstruction permits her to resume normal life activities at 3-days post-procedure.[97]
Tissue engineering: the breast mound
The breast-tissue matrix consists of engineered tissues of complex, implanted, biocompatible scaffolds seeded with the appropriate cells. The in-situ creation of a tissue matrix in the breast mound is begun with the external vacuum expansion of the mastectomy defect tissues (recipient site), for subsequent seeding (injecting) with autologous fat grafts of adipocyte tissue. The study Tissue Engineering a Breast Mound by External Expansion & Autologous Fat Grafting (2010), reported that serial fat-grafting to a pre-expanded recipient site achieved (with a few 2-mm incisions and minimally invasive blunt-cannula injection procedures), a non-surgical outcome equivalent to a surgical breast reconstrcution by autologous-flap procedure. Technically, the external vacuum expansion of the recipient-site tissues created a skin envelope as it stretched the mastectomy scar, and so generated a fertile breast-tissue matrix to which were injected large-volume fat grafts (150–600 ml) to create a breast of natural form, look, and feel.[98]
The fat graft breast reconstructions for 33 women (47 breasts, 14 irradiated), whose clinical statuses ranged from 0-days to 30-years post-mastectomy, began with the pre-expansion of the breast mound (recipient site) with an external vacuum tissue-expander for 10 hours daily, for 10–30 days before the first grafting of autologous fat. The breast mound expansion was adequate when the mastectomy scar tissues stretched to create a 200–300 ml recipient matrix (skin envelope), that received a fat-suspension volume of 150–600 ml in each grafting session.[98]
At 1-week post-procedure, the patients resumed using the external vacuum tissue-expander for 10 hours daily, until the next fat grafting session; 2–5 outpatient procedures, 6–16 weeks apart, were required until the plastic surgeon and the patient were satisfied with the volume, form, and feel of the reconstructed breasts. The follow-up mammogram and MRI examinations found neither defects (necrosis) nor abnormalities (neoplasms). At 6-months post-procedure, the reconstructed breasts had a natural form, look, and feel, and the stable breast-volumes ranged 300–600 ml per breast. The post-procedure mammographies indicated normal, fatty breasts with well-vascularized fat, and few, scattered, benign oil cysts. The occurred complications included pneumothorax and transient cysts.[98]
Explantation deformity
The non-surgical fat graft replacement of breast implants (saline and silicone) resolves medical complications such as: capsular contracture, implant shell rupture, filler leakage (silent rupture), device deflation, and silicone-induced granulomas, which are medical conditions usually requiring re-operation and explantation (breast implant removal). The patient then has the option of surgical or non-surgical breast corrections, either replace the explanted breast implants or fat graft breast augmentation.[99] Breast implant replacement with autologous fat grafts requires the external vacuum pre-expansion of the recipient-site tissues (adipose, glandular) to create a breast-tissue matrix to augment with injections of breast-filler fat. Moreover, because fat grafts are biologically sensitive, they cannot survive in the empty implantation socket, instead, they are injected to and diffused within the breas-tissue matrix(recipient site), replacing approximately 50 per cent of the volume of the removed implant — as permanent breast augmentation. The outcome of the explantation correction is a bust of natural appearance; breasts of volume, form, and feel, that — although approximately 50 per cent smaller than the explanted breast size — are larger than the original breast size, pre-procedure.[99]
Breast augmentation
The outcome of a breast augmentation with fat-graft injections depends upon proper patient selection, preparation, and correct technique for recipient site expansion, and the harvesting, refining, and injecting of the autologous breast-filler fat. Technical success follows the adequate external vacuum expansion of the recipient-site tissues (matrix) before the injection of large-volume grafts (220–650 cc) of autologous fat to the breasts. After harvesting by liposuction, the breast-filler fat was obtained by low G-force syringe centrifugation of the harvested fat to separate it, by density, from the crystalloid component. The refined breast filler then was injected to the pre-expanded recipient site; post-procedure, the patient resumed continual vacuum expansion therapy upon the injected breast, until the next fat grafting session. The mean operating room (OR) time was 2-hours, and there occurred no incidences of infection, cysts, seroma, hematoma, or tissue necrosis.[100]
The breast-volume data reported in Breast Augmentation with Autologous Fat Grafting: A Clinical Radiological Study (2010) indicated a mean increase of 1.2-times the initial breast volume, at 6-months post-procedure. In a 2-year period, 25 patients underwent breast augmentation by fat graft injection; at 3-weeks pre-procedure, before the fat grafting to the breast-tissue matrix (recipient site), the patients were photographed, and examined via intravenous contrast MRI or 3-D volumetric imaging, or both. The breast-filler fat was harvested by liposuction (abdomen, buttocks, thighs), and yielded fat-graft volumes of 220–650 cc per breast. At 6-months post-procedure, the follow-up treatment included photographs, intravenous contrast MRI or 3-D volumetric imaging, or both. Each woman had an increased breast volume of 250 cc per breast, a mean volume increase confirmed by quantitative MRI analysis. The mean increase in breast volume was 1.2-times the initial breast volume measurements; the statistical difference between the pre-procedure and the 6-month post-procedure breast volumes was (P< 00.0000007); the percentage increase basis of the breast volume was 60–80 per cent of the initial, pre-procedure breast volume.[100]
Complications and limitations
In every surgical and non-surgical procedure, the risk of complications exists before, during, and after a procedure, and, given the sensitive biological nature of breast tissues (adipocyte, glandular), this is especially true in the case of fat graft breast augmentation. Despite its relative technical simplicity, the injection (grafting) technique for breast augmentation is accompanied by post-procedure complications — fat necrosis, calcification, and sclerotic nodules — which directly influence the technical efficacy of the procedure, and of achieving a successful outcome. The Chinese study Breast Augmentation by Autologous Fat-injection Grafting: Management and Clinical analysis of Complications (2009), reported that the incidence of medical complications is reduced with strict control of the injection-rate (cc/min.) of the breast-filler volume being administered, and by diffusing the fat-grafts in layers to allow their even distribution within the breast tissue matrix. The complications occurred to the 17-patient group were identified and located with 3-D volumetric and MRI visualizations of the breast tissues and of any sclerotic lesions and abnormal tissue masses (malignant neoplasm). According to the characteristics of the defect or abnormality, the sclerotic lesion was excised and liquefied fat was aspirated; the excised samples indicated biological changes in the intramammary fat grafts — fat necrosis, calcification, hyalinization, and fibroplasia.[101]
The complications associated with injecting fat grafts to augment the breasts are like, but less severe, than the medical complications associated with other types of breast procedure. Technically, the use of minuscule (2-mm) incisions and blunt-cannula injection much reduce the incidence of damaging the underlying breast structures (milk ducts, blood vessels, nerves). Injected fat-tissue grafts that are not perfused among the tissues can die, and result in necrotic cysts and eventual calcifications — medical complications common to breast procedures. Nevertheless, a contoured abdomen for the patient is an additional benefit derived from the liposuction harvesting of the adipocyte tissue injected to the breasts. (see abdominoplasty)
When the patient’s body has insufficient adipocyte tissue to harvest as injectable breast filler, a combination of fat grafting and breast implants might provide the desired outcome. Although non-surgical breast augmentation with fat graft injections is not associated with implant-related medical complications (filler leakage, deflation, visibility, palpability, capsular contracture), the achievable breast volumes are physically limited; the large-volume, global bust augmentations realised with breast implants are not possible with the method of structural fat grafting. Global breast augmentation contrasts with the controlled breast augmentation of fat-graft injection, in the degree of control that the plastic surgeon has in achieving the desired breast contour and volume. The controlled augmentation is realised by infiltrating and diffusing the fat grafts throughout the breast; and it is feather-layered into the adjacent pectoral areas until achieving the desired outcome of breast volume and contour. Nonetheless, the physical fullness-of-breast achieved with injected fat-grafts does not visually translate into the type of buxom fullness achieved with breast implants; hence, patients who had plentiful fat-tissue to harvest attained a maximum breast augmentation of one brassiėre cup-size in one session of fat grafting to the breast.[102]
Breast cancer
Detection
A contemporary woman’s life-time probability of developing breast cancer is approximately one in seven;[103] yet there is no causal evidence that fat grafting to the breast might be more conducive to breast cancer than are other breast procedures; because incidences of fat tissue necrosis and calcification occur in every such procedure: breast biopsy, implantation, radiation therapy, breast reduction, breast reconstruction, and liposuction of the breast. Nonetheless, detecting breast cancer is primary, and calcification incidence is secondary; thus, the patient is counselled to learn self-palpation of the breast and to undergo periodic mammographic examinations. Although the mammogram is the superior diagnostic technique for distinguishing among cancerous and benign lesions to the breast, any questionable lesion can be visualized ultrasonically and magnetically (MRI); biopsy follows any clinically suspicious lesion or indeterminate abnormality appeared in a radiograph.[104]
Therapy
Breast augmentation via autologous fat grafts allows the oncological breast surgeon to consider conservative breast surgery procedures that usually are precluded by the presence of alloplastic breast implants, e.g. lumpectomy, if cancer is detected in an implant-augmented breast. In previously augmented patients, aesthetic outcomes cannot be ensured without removing the implant and performing mastectomy.[105][106] Moreover, radiotherapy treatment is critical to reducing cancerous recurrence and to the maximal conservation of breast tissue; yet, ironically, radiotherapy of an implant-augmented breast much increases the incidence of medical complications — capsular contracture, infection, extrusion, and poor cosmetic outcome.[107]
Post-cancer breast reconstruction
After mastectomy, surgical breast reconstruction with autogenous skin flaps and with breast implants can produce subtle deformities and deficiencies resultant from such global breast augmentation, thus the breast reconstruction is incomplete. In which case, fat graft injection can provide the missing coverage and fullness, and might relax the breast capsule. The fat can be injected as either large grafts or as small grafts, as required to correct difficult axillary deficiencies, improper breast contour, visible implant edges, capsular contracture, and tissue damage consequent to radiation therapy.[107]
Thailand breast slap
Thailand breast slap is a method of supposedly enlarging the breasts which has been endorsed by the government of Thailand as an alternative to breast implants. It was proposed by Khemmikka Na Sonkhla, a beautician who claimed her grandmother used the procedure to enlarge her breasts. The procedure's proponents claim that it also reduces the risk of breast cancer. The Thai government has currently enrolled over 20 women in publicly-funded courses that teach the technique.[108] However, the technique has not been endorsed or proven to be effective by mainstream science outside Thailand. However, a Thai doctor conducted a six-month study that gave promising results; the doctor also encouraged young girls to boost their bust size by eating more.[109]
See also
- Breast ironing, a breast reduction method used in the African country of Cameroon.
- Breast fetishism
- Triactol
References
- ^ Arion HG (1965). "Retromammary Prosthesis". C R Societé Française de Gynécologie. 5.
- ^ Stevens WG, Hirsch EM, Stoker DA, Cohen R. (2006). "In vivo Deflation of Pre-filled Saline Breast Implants". Plastic Reconstruction Surgery. 118 (2): 347–349. doi:10.1097/01.prs.0000227674.65284.80. PMID 16874200.
{{cite journal}}: CS1 maint: multiple names: authors list (link) - ^ Cronin TD, Gerow FJ (1963). "Augmentation Mammaplasty: A New "natural feel" Prosthesis". Excerpta Medica International Congress Series. 66: 41.
- ^ Luu HM, Hutter JC, Bushar HF (1998). "A Physiologically based Pharmacokinetic Model for 2,4-toluenediamine Leached from Polyurethane foam-covered Breast Implants". Environ Health Perspect. 106 (7). Environmental Health Perspectives, Vol. 106, No. 7: 393–400. doi:10.2307/3434066. JSTOR 3434066. PMC 1533137. PMID 9637796.
{{cite journal}}: CS1 maint: multiple names: authors list (link) - ^ Hester TR Jr, Tebbetts JB, Maxwell GP (2001). "The Polyurethane-covered Mammary Prosthesis: Facts and Fiction (II): A Look Back and a "peek" Ahead". Clinical Plastic Surgery. 28 (3): 579–586. PMID 11471963.
{{cite journal}}: CS1 maint: multiple names: authors list (link) - ^ Brown MH, Shenker R, Silver SA (2005). "Cohesive Silicone Gel Breast Implants in Aesthetic and Reconstructive Breast Surgery". Plastic Reconstructive Surgery. 116 (3): 768–779, discussion 780–1. doi:10.1097/01.prs.0000176259.66948.e7. PMID 16141814.
{{cite journal}}: CS1 maint: multiple names: authors list (link) - ^ Fruhstorfer BH, Hodgson EL, Malata CM (2004). "Early Experience with Anatomical Soft Cohesive Silicone gel Prosthesis in Cosmetic and Reconstructive Breast Implant Surgery". Annals of Plastic Surgery. 53 (6): 536–542. doi:10.1097/01.sap.0000134508.43550.6f. PMID 15602249.
{{cite journal}}: CS1 maint: multiple names: authors list (link) - ^ Heden P, Jernbeck J, Hober M (2001). "Breast Augmentation with Anatomical Cohesive gel Implants: The World's Largest Current Experience". Clinical Plastic Surgery. 28 (3): 531–552. PMID 11471959.
{{cite journal}}: CS1 maint: multiple names: authors list (link) - ^ Breastfeeding after Breast Surgery, La Leche League, contains numerous references.
- ^ Breastfeeding and Breast Implants, Selected Bibliography April 2003, LLLI Center for Breastfeeding Information
- ^ a b c Inorganic Milk: Can Kendra Wilkinson breast-feed her baby even though she has implants?, Christopher Beam, Slate.com, December 11, 2009
- ^ Berlin, C. M. Silicone breast implants and breast-feeding. Pediatrics 1994; 94:546-49.
- ^ a b Silicone Breast Implants and Breastfeeding, Cheston M. Berlin, Jr. MD, Hershey Medical Center, Hershey, PA, from Breastfeeding Abstracts, February 1996, Volume 15, Number 3, pp. 17-18.
- ^ Handel, N., Silverstein, M. J., Gamagami, P., Jensen, J. A., and Collins, A. (1992). "actors affecting mammographic visualization of the breast after augmentation mammaplasty". JAMA. 268 (14): 1913–1917. doi:10.1001/jama.268.14.1913. PMID 1404718.
{{cite journal}}: CS1 maint: multiple names: authors list (link) - ^ Clark CP 3rd, Peters GN, O'Brien KM. (1993). "Cancer in the augmented breast. Diagnosis and prognosis". Cancer. 72 (7): 2170–4. doi:10.1002/1097-0142(19931001)72:7<2170::AID-CNCR2820720717>3.0.CO;2-1. PMID 8374874.
{{cite journal}}: CS1 maint: multiple names: authors list (link) CS1 maint: numeric names: authors list (link) - ^ Skinner KA, Silberman H, Dougherty W, Gamagami P, Waisman J, Sposto R, Silverstein MJ. (2001). "Breast cancer after augmentation mammoplasty". Ann Surg Oncol. 8 (2): 138–44. doi:10.1007/s10434-001-0138-x. PMID 11258778.
{{cite journal}}: CS1 maint: multiple names: authors list (link) - ^ Le GM, O'Malley CD, Glaser SL, Lynch CF, Stanford JL, Keegan TH, West DW. (2005). "Breast implants following mastectomy in women with early-stage breast cancer: prevalence and impact on survival". Breast Cancer Res. 7 (2): R184–93. doi:10.1186/bcr974. PMC 1064128. PMID 15743498.
{{cite journal}}: CS1 maint: multiple names: authors list (link) CS1 maint: unflagged free DOI (link) - ^ a b Handel N, Silverstein MJ (2006). "Breast cancer diagnosis and prognosis in augmented women". Plastic Reconstructive Surgery. 118 (3): 587–93. doi:10.1097/01.prs.0000233038.47009.04. PMID 16932162. Cite error: The named reference "Handel2006" was defined multiple times with different content (see the help page).
- ^ Cunningham B (2006). "Breast cancer diagnosis and prognosis in augmented women- Discussion". Plastic Reconstructive Surgery. 118: 594–95. doi:10.1097/01.prs.0000233047.87102.8e. PMID 16932163.
- ^ SChwartz GF; et al. (2006). "Consensus Conference on Breast Conservation". JACAS. 203 (2): 198–207. doi:10.1016/j.jamcollsurg.2006.04.009. PMID 16864033.
{{cite journal}}: Explicit use of et al. in:|author=(help) - ^ Johnson GW, Christ JE. (1993). "The Endoscopic Breast augmentation: The Transumbilical Insertion of Saline-filled Breast Implants". Plastic Reconstructive Surgery. 92 (5): 801–808. PMID 8415961.
- ^ Wallach SG. (2004). "Maximizing the Use of the Abdominoplasty Incision". Plastic Reconstruction Surgery. 113 (1): 411–417. doi:10.1097/01.PRS.0000091422.11191.1A. PMID 14707667.
- ^ Graf RM; et al. (2003). "Subfascial Breast Implant: A New Procedure". Plastic Reconstructive Surgery. 111 (2): 904–908. doi:10.1097/01.PRS.0000041601.59651.15. PMID 12560720.
{{cite journal}}: Explicit use of et al. in:|author=(help) - ^ Tebbetts JB (2004). "Does Fascia Provide Additional, Meaningful Coverage over a Breast Implant?". Plastic Reconstructive Surgery. 113 (2): 777–79. doi:10.1097/01.PRS.0000104516.13465.96. PMID 14758271.
- ^ Tebbetts T (2002). "A System for Breast Implant Selection Based on Patient Tissue Characteristics and Implant-soft tissue Dynamics". Plastic Reconstructive Surgery. 109 (4): 1396–1409. doi:10.1097/00006534-200204010-00030. PMID 11964998.
- ^ Pacik, P.T. “Pain Control in Augmentation Mammaplasty: Safety and Efficacy of Indwelling Catheters in 644 Consecutive Patients” Aesthetic Surgery Journal, 28:279–84, May/June 2008
- ^ Pacik, P.T. “Pain Control in Augmentation Mammaplasty Using Indwelling Catheters in 687 Consecutive Patients: Data Analysis”. Aesthetic Surgery Journal, 28:631–41, November/December 2008
- ^ "Important Information for Women About Breast Augmentation with INAMED Silicone-Filled Breast Implants" (PDF). 2006-11-03. Retrieved 2007-05-04.
- ^ "Important Information for Augmentation Patients About Mentor MemoryGel Silicone Gel-Filled Breast Implants" (PDF). 2006-11-03. Retrieved 2007-05-04.
- ^ a b c "Local Complications". FDA Breast Implant Consumer Handbook - 2004. 2004-06-08. Retrieved 2007-05-04.
- ^ Tebbets JB (2006). "Out points" criteria for breast implant removal without replacement and criteria to minimize reoperations following breast augmentation". Plastic Reconstructive Surgery. 114 (5): 1258–62. PMID 15457046.
{{cite journal}}: Unknown parameter|month=ignored (help) - ^ Tebbets JB (2006). "Achieving a zero percent reoperation rate at 3 years in a 50-consecutive-case augmentation mammaplasty premarket approval study". Plastic Reconstructive Surgery. 118 (6): 1453–7. doi:10.1097/01.prs.0000239602.99867.07. PMID 17051118.
{{cite journal}}: Unknown parameter|month=ignored (help) - ^ Brown SL, Middleton MS, Berg WA, Soo MS, Pennello G (2000). "Prevalence of Rupture of Silicone gel Breast Implants Revealed on MR Imaging in a Population of Women in Birmingham, Alabama". American Journal of Roentgenology. 175 (4): 1057–64. PMID 11000165.
{{cite journal}}: CS1 maint: multiple names: authors list (link) - ^ Walker PS; et al. (2009). "Natrelle Saline-filled Breast Implants: a Prospective 10-year Study". Aesthetic Surgery Journal. 29 (1): 19–25. doi:10.1016/j.asj.2008.10.001. PMID 19233001.
{{cite journal}}: Explicit use of et al. in:|author=(help) - ^ Holmich LR; et al. (2004). "Untreated Silicone Breast Implant Rupture". Plastic Reconstructive Surgery. 114 (1): 204–14. doi:10.1097/01.PRS.0000128821.87939.B5. PMID 15220594.
{{cite journal}}: Explicit use of et al. in:|author=(help) - ^ Katzin, William E, Ceneno, Jose A, Feng, Lu-Jean; et al. (2001). "Pathology of Lymph Nodes From Patients With Breast Implants: A Histologic and Spectroscopic Evaluation" (—Scholar search). American Journal of Surgical Pathology. 29 (4): 506–11. PMID 15767806.
{{cite journal}}: Explicit use of et al. in:|author=(help); External link in|format= - ^ "Study of Rupture of Silicone Gel-filled Breast Implants (MRI Component)". FDA Breast Implant Consumer Handbook - 2004. 2000-05-22. Retrieved 2007-05-04.
- ^ Holmich LR; et al. (2003). "Incidence of Silicone Breast Implant Rupture". Arch Surg. 138 (7): 801–06. doi:10.1001/archsurg.138.7.801. PMID 12860765.
{{cite journal}}: Explicit use of et al. in:|author=(help) - ^ Heden P; et al. (2006). "Prevalence of Rupture in Inamed Silicone Breast Implants". Plastic Reconstructive Surgery. 118 (2): 303–8. doi:10.1097/01.prs.0000233471.58039.30. PMID 16874191.
{{cite journal}}: Explicit use of et al. in:|author=(help) - ^ "FDA summary of clinical issues (MS Word document)".
- ^ Cunningham, B; et al. (2009). "Safety and effectiveness of Mentor's MemoryGel implants at 6 years". Plastic Reconstructive Surgery. 33 (3): 440–44. doi:10.1007/s00266-009-9364-6. PMID 19437068.
{{cite journal}}: Explicit use of et al. in:|author=(help) - ^ Heden P; et al. (2006). "Style 410 Cohesive Silicone Breast Implants: Safety and Effectiveness at 5 to 9 years after Implantation". Plastic Reconstructive Surgery. 118 (6): 1281–1287. doi:10.1097/01.prs.0000239457.17721.5d. PMID 17051096.
{{cite journal}}: Explicit use of et al. in:|author=(help) - ^ Barnsley GP; Sigurdson, LJ; Barnsley, SE (2006). "Textured surface breast implants in the prevention of capsular contracture among breast augmentation patients: a meta-analysis of randomized controlled trials". Plastic Reconstructive Surgery. 117 (7): 2182–90. doi:10.1097/01.prs.0000218184.47372.d5. PMID 16772915.
- ^ Wong CH, Samuel M, Tan BK, Song C. (2006). "Capsular contracture in subglandular breast augmentation with textured versus smooth breast implants: a systematic review". Plastic Reconstructive Surgery. 118 (5): 1224–1236. doi:10.1097/01.prs.0000237013.50283.d2. PMID 17016195.
{{cite journal}}: CS1 maint: multiple names: authors list (link) - ^ Mladick RA (1993). ""No-touch" submuscular saline breast augmentation technique". Journal of Aesthetic Surgery. 17 (3): 183–92. doi:10.1007/BF00636260. PMID 8213311.
- ^ Adams WP jr.; et al. (2006). "Enhancing patient outcomes in aesthetic and reconstructive breast surgery using triple antibiotic breast irrigation: six-year prospective clinical study". Plastic Reconstructive Surgery. 117 (1): 30–36. doi:10.1097/01.prs.0000208306.79104.18. PMID 16404244.
{{cite journal}}: Explicit use of et al. in:|author=(help) - ^ Planas J; Cervelli, V; Planas, G (2001). "Five-year experience on ultrasonic treatment of breast contractures". Aesthetic Plastic Surgery. 25 (2): 89–93. doi:10.1007/s002660010102. PMID 11349308.
- ^ Schlesinger SL, wt al (2002). "Zafirlukast (Accolate): A new treatment for capsular contracture". Aesthetic Plast Surg. 22 (4): 329–36.
- ^ Scuderi N; et al. (2006). "The effects of zafirlukast on capsular contracture: preliminary report". Aesthetic Plast Surg. 30 (5): 513–20. doi:10.1007/s00266-006-0038-3. PMID 16977359.
{{cite journal}}: Explicit use of et al. in:|author=(help) - ^ Silver H (1982). "Reduction of capsular contracture with two-stage augmentation mammaplasty and pulsed electromagnetic energy (Diapulse therapy)". Plastic Reconstructive Surgery. 69 (5): 802–08. doi:10.1097/00006534-198205000-00013. PMID 7071225.
- ^ a b Rinzler, Carol Ann (2009) The encyclopedia of Cosmetic and Plastic Surgery New York:Facts on File, p.23.
- ^ Arepelli S; et al. (2002). "Allergic reactions to platinum in silicone breast implants". J Long-Term Effects Medical Implants. 12 (4): 299–306. PMID 12627791.
{{cite journal}}: Explicit use of et al. in:|author=(help) - ^ Therapeutic Goods Administration (2001). Breast Implant Information Booklet (PDF) (4th ed.). Australian Government. ISBN 0-642-73579-4.
- ^ European Committee on Quality Assurance and Medical Devices in Plastic Surgery (2000-06-23). "Consensus Declaration on Breast Implants" (PDF). Archived from the original (PDF) on December 30, 2006. Retrieved 2007-05-04.
- ^ "Silicone Gel Breast Implant Report Launched - No Epidemiological Evidence For Link With Connective Tissue Disease - Independent Review Group". 1998-07-13. Retrieved 2007-05-04.
- ^ "Expert Advisory Panel on Breast Implants: Record of Proceedings". HealthCanada. 2005-09-29. Retrieved 2007-05-04.
- ^ Vasey FB, Zarabadi SA, Seleznick M, Ricca L (2003). "Where there's Smoke there's Fire: the silicone breast implant controversy continues to flicker: a new disease that needs to be defined". Journal of Rheumatology. 30 (10): 2092–2094. PMID 14528500.
{{cite journal}}: CS1 maint: multiple names: authors list (link) - ^ Breiting VB, Holmich LR, Brandt B, Fryzek JP, Wolthers MS, Kjoller K, McLaughlin JK, Wiik A, Friis S (2004). "Long-term Health Status of Danish Women with Silicone Breast Implants". Plastic and Reconstructive Surgery. 114 (1): 217–226. doi:10.1097/01.PRS.0000128823.77637.8A. PMID 15220596.
{{cite journal}}: CS1 maint: multiple names: authors list (link) - ^ Kjoller K, Holmich LR, Fryzek JP, Jacobsen PH, Friis S, McLaughlin JK, Lipworth L, Henriksen TF, Hoier-Madsen M, Wiik A, Olsen JH (2004). "Self-reported musculoskeletal symptoms among Danish women with cosmetic breast implants". Plastic Ann Plast Surg. 52 (1): 1–7. doi:10.1097/01.sap.0000101930.75241.55. PMID 14676691.
{{cite journal}}: CS1 maint: multiple names: authors list (link) - ^ Fryzek JP, Holmich L, McLaughlin JK, Lipworth L, Tarone RE, Henriksen T, Kjoller K, Friis S (2007). "A Nationwide Study of Connective Tissue Disease and Other Rheumatic Conditions Among Danish Women With Long-Term Cosmetic Breast Implantation". Ann Epidemiol. 17 (5): 374–9. doi:10.1016/j.annepidem.2006.11.001. PMID 17321754.
{{cite journal}}: CS1 maint: multiple names: authors list (link) - ^ Brinton LA, Lubin JH, Murray MC, Colton T, Hoover RN (2006). "Mortality Rates Among Augmentation Mammoplasty patients: an update". Epidemiology. 17 (2): 162–9. doi:10.1097/01.ede.0000197056.84629.19. PMID 16477256.
{{cite journal}}: CS1 maint: multiple names: authors list (link) - ^ McLaughlin JK, Lipworth L, Fryzek JP, Ye W, Tarone RE, Nyren O (2006). "Long-term Cancer Risk Among Swedish Women with Cosmetic Breast Implants: an update of a nationwide study". J Natl Cancer Inst. 98 (8): 557–60. doi:10.1093/jnci/djj134. PMID 16622125.
{{cite journal}}: CS1 maint: multiple names: authors list (link) - ^ Villenueve PJ; et al. (2006). "Mortality among Canadian Women with Cosmetic Breast Implants". American Journal of Epidimiology. 164 (4): 334–41. doi:10.1093/aje/kwj214. PMID 16777929.
{{cite journal}}: Explicit use of et al. in:|author=(help); Unknown parameter|month=ignored (help) - ^ Brown SL, Pennello G, Berg WA, Soo MS, Middleton MS (2001). "Silicone gel Breast Implant Rupture, Extracapsular Silicone, and Health Status in a Population of Women". Journal of Rheumatology. 28 (5): 996–1003. PMID 11361228.
{{cite journal}}: CS1 maint: multiple names: authors list (link) - ^ Lipworth L, Tarone RE, McLaughlin JK. (2004). "Breast Implants and Fibromyalgia: a Review of the Epidemiologic Evidence". Annals of Plastic Surgery. 52 (3): 284–87. doi:10.1097/01.sap.0000116024.18713.28. PMID 15156983.
{{cite journal}}: CS1 maint: multiple names: authors list (link) - ^ a b "Diseases". FDA Breast Implant Consumer Handbook - 2004. 2004-06-08. Retrieved 2007-05-04.
- ^ Fischer G. Surgical Treatment of Cellulitis (presentation) Third Congress of the International Academy of Cosmetic Surgery, Rome, 31 May 1975
- ^ Fischer G. First Surgical Treatment for Modelling the Body’s Cellulite with Three 5-mm incisions. Bulletin of the International Academy of Cosmetic Surgery 1976; 2:35–37
- ^ Fischer A., Fischer G. Revised Technique for Cellulite fat reduction in Riding Breeches deformity. Bulletin of the International Academy of Cosmetic Surgery 1977; 2(4):40–43
- ^ Schiffman MA. (2010) Autologous Fat Transfer: Art, Science, and Clinical Practice Springer Verlag: Berlin, Heidelberg, p. 3
- ^ Bircoll M. Autologous Fat Transplantation (presentation) The Asian Congress of Plastic Surgery, February 1982
- ^ Bircoll MJ. New Frontiers in Suction Lipectomy (presentation) Second Asian Congress of Plastic Surgery, Pattiya, Thailand, February 1984
- ^ Krulig E. Lipo-injection. American Journal of Cosmetic Surgery 1987; 4(2): 123–129
- ^ Schiffman MA. (2010) Autologous Fat Transfer: Art, Science, and Clinical Practice Springer Verlag: Berlin, Heidelberg, p. 4
- ^ Newman J, Levin J. Facial Lipo-transplant Surgery. American Journal of Cosmetic Surgery 1987; 4(2):131–140
- ^ Agris J. Autologous Fat Transplantation: A Three-year Study. American Journal of Cosmetic Surgery 1987; 4(2): 95–102
- ^ Schiffman MA. (2010) Autologous Fat Transfer: Art, Science, and Clinical Practice Springer Verlag: Berlin, Heidelberg, p. 226
- ^ Pierrefeu-Lagrange, A. C., Delay, E., Guerin, N., et al. Radiological evaluation of breasts reconstructed with lipo-modeling (in French). Annales de Chirurgie Plastique Esthétique 51:18, 2005
- ^ Zocchi, M. L., Zuliani, F., Nava, M., et al. Bicompartmental breast lipostructuring (presentation) 7th International Congress of Aesthetic Medicine, Milan, 13–15 October 2005
- ^ Rigotti, G., Marchi, A., Galiè, M., et al. Clinical treatment of radiotherapy tissue damages by lipoaspirates transplant: A healing process mediated by adipose-derived stem cells (ASCS). Plastic and Reconstructive Surgery (accepted for publication)
- ^ Holle, J. Lipofilling in rhinoplasty and breast augmentation (presentation) American Alpine Workshop in Plastic Surgery 17th Annual Meeting, Sun Valley, Idaho, 12–17 February 2006
- ^ Massiha, H. Scar-tissue flaps for the correction of post-implant breast rippling. Annals of Plastic Surgery 48:505, 2002
- ^ Baruffaldi-Preis, F. La correzione delle depressioni: Esiti cicatriziali e rippling (presentatio) 30th Anniversary Course of the Foundation of G. Sanvenero Rosselli, Milan, Italy, 16 September 2005
- ^ Coleman SR and Sabeiro AP. Fat Grafting to the breast Revisited: Safety and Efficacy. Plastic and Reconstructive Surgery 2007; 119(3):775–785
- ^ a b Coleman SR, Saboeiro AP, Fat Grafting to the Breast Revisited: Safety and Efficacy, Plastic and Reconstructive Surgery March 2007 p. 776
- ^ Asken S. Autologous Fat Transplantation: Micro and Macro Techniques. American Journal of Cosmetic Surgery 1987; 4(2):111–121
- ^ Toledo LS. Syringe Liposculpture: A Two-year Experience. Aesthetic Plastic Surgery 1991;15(4:321–326
- ^ Uebel CO. Facial Sculpture with Centrifuged fat Collagen, Hinder VT (ed.) Plastic Surgery Vol. II. Amsterdam Excerpta Medica 1992 pp. 749–752
- ^ Schiffman MA. (2010) Autologous Fat Transfer: Art, Science, and Clinical Practice Springer Verlag: Berlin, Heidelberg, p. 5
- ^ Coleman SR. Avoidance of Arterial Occlusion from Injection of Soft tissue Fillers. Aesthetic Surgery Journal 22:555,2002. Plastic and Reconstructive Surgery. March 2007
- ^ Guerney CE. Experimental Study of the Behavior of Free fat Transplants. Surgery 1938, 3:679–692
- ^ Coleman SR, Saboeiro AP, Fat Grafting to the Breast Revisited: Safety and Efficacy. Plastic and Reconstructive Surgery. March 2007 p. 779
- ^ Neuhof H. (1923) The Transplantation of Tissues New York:D. Appleton p. 74
- ^ Rigotti G, Marchi A, Galiè M. et al. Clinical Treatment of Radiotherapy Tissue Damages by Lipoaspirates Transplant: a Healing Process Mediated by Adipose-derived stem cells (ASCS). Plastic and Reconstructive Surgery (accepted for publication).
- ^ a b Khouri RK, Schlenz I, Murphy BJ, Baker TJ. Non-surgical Breast Enlargement using an External Soft Tissue Expansion System. Plastic and Reconstructive Surgery 2000 June; 105(7): 2500–2512; discussion 2513–2534
- ^ a b Del Vecchio DA, Bucky LP. Breast Augmentation using Pre-expansion and Autologous fat Transplantation — A Clinical Radiological Study. Plastic and Reconstructive Surgery 2011 February 8
- ^ a b c Khouri RK (2010) Non-surgical breast reconstruction with autologous fat-grafts
- ^ a b c Khouri RK, Cardoso E, Marchi A, Rigotti G. (2010) Tissue Engineering a Breast Mound by External expansion & Autologous fat Grafting
- ^ a b Khouri, R.K. (2010) Breast Implants Problems and Risks
- ^ a b Del Vecchio D, Bucky LP. Breast Augmentation with Autologous Fat Grafting: A Clinical Radiological Study. Plastic and Reconstructive Surgery. October 2010; 126: pp. 68–69
- ^ Mu DL, Luan J, Mu L, Xin MQ. Breast Augmentation by Autologous Fat injection grafting: Management and Clinical Analysis of Complications. Annals of Plastic Surgery 2009 Aug; 63(2):124–127
- ^ Coleman SR, Saboeiro AP, Fat Grafting to the Breast Revisited: Safety and Efficacy, Plastic and Reconstructive Surgery March 2007 p. 782
- ^ Gloeckler Ries, L. A., Reichman, M. E., Lewis, D. R., et al. Cancer survival and incidence from the Surveillance, Epidemiology, and End Results (SEER) program. Oncologist 8:541, 2003
- ^ Coleman SR, Saboeiro AP, Fat Grafting to the Breast Revisited: Safety and Efficacy, Plastic and Reconstructive Surgery March 2007 pp. 782–783
- ^ Karanas YL, Leong DS, da Lio A et al. Surgical Treatment of Breast Cancer in Previously Augmented Patients. Plastic and Reconstructive Surgery. 2003 111:1078
- ^ Handel N, Lewinsky B, Jensen J A et al. Breast Conservation Therapy after Augmentation Mammaplasty: Is it Appropriate? Plastic and Reconstructive Surgery. 1996 98:1216
- ^ a b Coleman SR, Saboeiro AP, Fat Grafting to the Breast Revisited: Safety and Efficacy. Plastic and Reconstructive Surgery. March 2007 pp. 782–783
- ^ http://thsnews.com/thailand-breast-slap-government-sponsors-bust-enlargement-program/324640/
- ^ McGirk, Jan (February 22, 2003). "Thailand sponsors slapping to enhance breast size".
