Prolyl endopeptidase
| prolyl oligopeptidase | |||||||||
|---|---|---|---|---|---|---|---|---|---|
| Identifiers | |||||||||
| EC no. | 3.4.21.26 | ||||||||
| CAS no. | 72162-84-6 | ||||||||
| Databases | |||||||||
| IntEnz | IntEnz view | ||||||||
| BRENDA | BRENDA entry | ||||||||
| ExPASy | NiceZyme view | ||||||||
| KEGG | KEGG entry | ||||||||
| MetaCyc | metabolic pathway | ||||||||
| PRIAM | profile | ||||||||
| PDB structures | RCSB PDB PDBe PDBsum | ||||||||
| Gene Ontology | AmiGO / QuickGO | ||||||||
| |||||||||
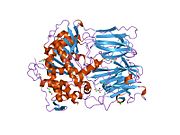
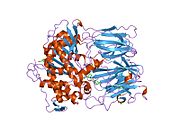
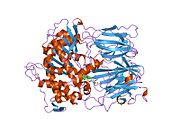
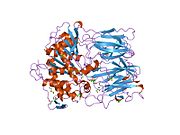
Prolyl endopeptidase (PREP) or prolyl oligopeptidase, sometimes post-proline cleaving enzyme) is a large cytosolic enzyme that belongs to a distinct class of serine peptidases. It was first described in the cytosol of rabbit brain as an oligopeptidase, which degrades the nonapeptide bradykinin at the Pro-Phe bond.[1] The enzyme is involved in the maturation and degradation of peptide hormones and neuropeptides such as alpha-melanocyte-stimulating hormone, luteinizing hormone-releasing hormone (LH-RH), thyrotropin-releasing hormone, angiotensin, neurotensin, oxytocin, substance P and vasopressin. PREP cleaves peptide bonds at the C-terminal side of proline residues. Its activity is confined to action on oligopeptides of less than 10 kD and it has an absolute requirement for the trans-configuration of the peptide bond preceding proline.
Some types of prolyl endopeptidase have been used in studies to decrease the propensity of gluten-containing wheat products to aggravate coeliac disease.[2] However, orally administered enzymes are potentially subject to inactivation in the gastrointestinal tract.[3]
In humans, prolyl endopeptidase is encoded by the PREP gene.[4][5] The protein encoded by this gene is a cytosolic prolyl endopeptidase that cleaves peptide bonds on the C-terminal side of prolyl residues within peptides that are up to approximately 30 amino acids long. Only short protein residues are able to enter the active site of prolyl endopeptidase due to the distinct beta-propeller region that acts as a gating filter mechanism.[6][7] Prolyl endopeptidases have been reported to be involved in the maturation and degradation of peptide hormones and neuropeptides.[5] There's an indication that altered PREP activity is associated with autism spectrum disorders and various psychological diseases such as schizophrenia, mania and clinical depression.[8]
Inhibitors
Several prolyl endopeptidase inhibitors are known,[9][10] and have been suggested as possible nootropic and antidepressant drugs.[11][12] Notable compounds include
References
- ^ Oliveira EB, Martins AR, Camargo ACM (1976). "Isolation of brain endopeptidases: Influence of size and sequence of substrates structurally related to bradykinin". Biochemistry. 15 (9): 1967–74. doi:10.1021/bi00654a026. PMID 5120.
{{cite journal}}: Unknown parameter|month=ignored (help)CS1 maint: multiple names: authors list (link) - ^ Stepniak D, Spaenij-Dekking L, Mitea C; et al. (2006). "Highly efficient gluten degradation with a newly identified prolyl endoprotease: implications for celiac disease". Am J Physiol Gastrointest Liver Physiol. 291 (4): G621–9. doi:10.1152/ajpgi.00034.2006. PMID 16690904.
{{cite journal}}: Explicit use of et al. in:|author=(help); Unknown parameter|month=ignored (help)CS1 maint: multiple names: authors list (link) - ^ Fuhrmann G, Leroux JC (2011) In vivo fluorescence imaging of exogenous enzyme activity in the gastrointestinal tract. Proceedings of the National Academy of Sciences, 108, 9032-9037 [1]
- ^ Vanhoof G, Goossens F, Hendriks L, De Meester I, Hendriks D, Vriend G, Van Broeckhoven C, Scharpe S (1994). "Cloning and sequence analysis of the gene encoding human lymphocyte prolyl endopeptidase". Gene. 149 (2): 363–6. doi:10.1016/0378-1119(94)90177-5. PMID 7959018.
{{cite journal}}: Unknown parameter|month=ignored (help)CS1 maint: multiple names: authors list (link) - ^ a b "Entrez Gene: PREP prolyl endopeptidase".
- ^ V. Fulop, Z. Bocskei, L. Polgar (1998). "Prolyl Oligopeptidase: An Unusual b-Propeller Domain Regulates Proteolysis" (PDF). Cell. 94: 161.
{{cite journal}}: CS1 maint: multiple names: authors list (link) - ^ V. Fulop, Z. Szeltner, L. Polgar (2000). "Catalysis of serine oligopeptidases is controlled by a gating filter mechanism". EMBO Rep. 1 (3): 277.
{{cite journal}}: CS1 maint: multiple names: authors list (link) - ^ Momeni N, Nordström BM, Horstmann V, Avarseji H, Sivberg BV (2005). "Alterations of prolyl endopeptidase activity in the plasma of children with autistic spectrum disorders". BMC Psychiatry. 5: 27. doi:10.1186/1471-244X-5-27. PMC 1190193. PMID 15932649.
{{cite journal}}: CS1 maint: multiple names: authors list (link) CS1 maint: unflagged free DOI (link) - ^ Jarho EM, Venäläinen JI, Poutiainen S; et al. (2007). "2(S)-(Cycloalk-1-enecarbonyl)-1-(4-phenyl-butanoyl)pyrrolidines and 2(S)-(aroyl)-1-(4-phenylbutanoyl)pyrrolidines as prolyl oligopeptidase inhibitors". Bioorg. Med. Chem. 15 (5): 2024–31. doi:10.1016/j.bmc.2006.12.036. PMID 17215128.
{{cite journal}}: Explicit use of et al. in:|author=(help); Unknown parameter|month=ignored (help)CS1 maint: multiple names: authors list (link) - ^ Kánai K, Arányi P, Böcskei Z; et al. (2008). "Prolyl oligopeptidase inhibition by N-acyl-pro-pyrrolidine-type molecules". J. Med. Chem. 51 (23): 7514–22. doi:10.1021/jm800944x. PMID 19006380.
{{cite journal}}: Explicit use of et al. in:|author=(help); Unknown parameter|month=ignored (help)CS1 maint: multiple names: authors list (link) - ^ Morain P, Boeijinga PH, Demazières A, De Nanteuil G, Luthringer R (2007). "Psychotropic profile of S 17092, a prolyl endopeptidase inhibitor, using quantitative EEG in young healthy volunteers". Neuropsychobiology. 55 (3–4): 176–83. doi:10.1159/000107070. PMID 17700042.
{{cite journal}}: CS1 maint: multiple names: authors list (link) - ^ Khlebnikova NN, Krupina NA, Bogdanova NG, Zolotov NN, Kryzhanovskii GN (2009). "Effects of prolylendopeptidase inhibitor benzyloxycarbonyl-methionyl-2(S)-cyanopyrrolidine on experimental depressive syndrome development in rats". Bull. Exp. Biol. Med. 147 (1): 26–30. PMID 19526123.
{{cite journal}}: Unknown parameter|month=ignored (help)CS1 maint: multiple names: authors list (link) - ^ Yoshimoto T, Kado K, Matsubara F, Koriyama N, Kaneto H, Tsura D. "Pramiracetam, a nootropic drug, is a prolyl oligopeptidase inhibitor". J Pharmacobiodyn.
{{cite journal}}: CS1 maint: multiple names: authors list (link) - ^ Tarragó T, Kichik N, Claasen B, Prades R, Teixidó M, Giralt E (2008). "Baicalin, a prodrug able to reach the CNS, is a prolyl oligopeptidase inhibitor". Bioorg. Med. Chem. 16 (15): 7516–24. doi:10.1016/j.bmc.2008.04.067. PMID 18650094.
{{cite journal}}: Unknown parameter|month=ignored (help)CS1 maint: multiple names: authors list (link) - ^ Toide K, Shinoda M, Miyazaki A (1998). "A novel prolyl endopeptidase inhibitor, JTP-4819--its behavioral and neurochemical properties for the treatment of Alzheimer's disease". Rev Neurosci. 9 (1): 17–29. PMID 9683325.
{{cite journal}}: CS1 maint: multiple names: authors list (link) - ^ Jalkanen AJ, Puttonen KA, Venäläinen JI, Sinervä V, Mannila A, Ruotsalainen S, Jarho EM, Wallén EA, Männistö PT (2007). "Beneficial effect of prolyl oligopeptidase inhibition on spatial memory in young but not in old scopolamine-treated rats". Basic Clin. Pharmacol. Toxicol. 100 (2): 132–8. doi:10.1111/j.1742-7843.2006.00021.x. PMID 17244263.
{{cite journal}}: Unknown parameter|month=ignored (help)CS1 maint: multiple names: authors list (link) - ^ Morain P, Lestage P, De Nanteuil G, Jochemsen R, Robin JL, Guez D, Boyer PA (2002). "S 17092: a prolyl endopeptidase inhibitor as a potential therapeutic drug for memory impairment. Preclinical and clinical studies". CNS Drug Rev. 8 (1): 31–52. PMID 12070525.
{{cite journal}}: CS1 maint: multiple names: authors list (link)
Further reading
External links
- prolyl+endopeptidase,+human at the U.S. National Library of Medicine Medical Subject Headings (MeSH)
- Prolyl Endopeptidase entry at the National Center for Biotechnology Information