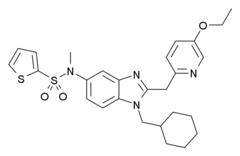
AZ-11713908
Appearance
 | |
| Identifiers | |
|---|---|
| |
| CAS Number | |
| ChemSpider | |
| Chemical and physical data | |
| Formula | C27H32N4O3S2 |
| Molar mass | 524.697 g/mol g·mol−1 |
| 3D model (JSmol) | |
| |
| |
| | |
AZ-11713908 is a drug developed by AstraZeneca which is a peripherally selective cannabinoid agonist, acting as a potent agonist at the CB1 receptor and a partial agonist at CB2. It has poor blood–brain barrier penetration, and so while it is an effective analgesic in animal tests, it produces only peripheral effects at low doses, with much weaker symptoms of central effects compared to other cannabinoid drugs such as WIN 55,212-2.[1] A large number of related benzimidazole derived cannabinoid ligands are known.[2][3][4][5]
See also
References
- ^ Yu XH, Cao CQ, Martino G, Puma C, Morinville A, St-Onge S, Lessard E, Perkins MN, Laird JM (November 2010). "A peripherally restricted cannabinoid receptor agonist produces robust anti-nociceptive effects in rodent models of inflammatory and neuropathic pain". Pain. 151 (2): 337–44. doi:10.1016/j.pain.2010.07.019. PMID 20696525.
{{cite journal}}: CS1 maint: multiple names: authors list (link) - ^ Verbist BM, De Cleyn MA, Surkyn M, Fraiponts E, Aerssens J, Nijsen MJ, Gijsen HJ (April 2008). "5-Sulfonyl-benzimidazoles as selective CB2 agonists". Bioorganic & Medicinal Chemistry Letters. 18 (8): 2574–9. doi:10.1016/j.bmcl.2008.03.048. PMID 18394887.
{{cite journal}}: CS1 maint: multiple names: authors list (link) - ^ Pagé D, Balaux E, Boisvert L, Liu Z, Milburn C, Tremblay M, Wei Z, Woo S, Luo X, Cheng YX, Yang H, Srivastava S, Zhou F, Brown W, Tomaszewski M, Walpole C, Hodzic L, St-Onge S, Godbout C, Salois D, Payza K, Payza K (July 2008). "Novel benzimidazole derivatives as selective CB2 agonists". Bioorganic & Medicinal Chemistry Letters. 18 (13): 3695–700. doi:10.1016/j.bmcl.2008.05.073. PMID 18522867.
{{cite journal}}: CS1 maint: multiple names: authors list (link) - ^ WO patent 2004/108688, LIU Z, PAGÈ D, WALPOLE C, YANG H, "BENZIMIDAZOLE DERIVATIVES, COMPOSITIONS CONTAINING THEM, PREPARATION THEREOF AND USES THEREOF", granted 16.12.2004
- ^ WO patent 2004/108712, LIU Z, PAGÈ D, WALPOLE C, YANG H, "BENZIMIDAZOLE DERIVATIVES, COMPOSITIONS CONTAINING THEM, PREPARATION THEREOF AND USES THEREOF", granted 16.12.2004
