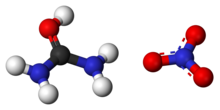
Urea nitrate

| |
| |
| Identifiers | |
|---|---|
3D model (JSmol)
|
|
| ChemSpider | |
| ECHA InfoCard | 100.004.276 |
PubChem CID
|
|
CompTox Dashboard (EPA)
|
|
| |
| |
| Properties | |
| CH5N3O4 | |
| Molar mass | 123.068 g/mol |
| Density | 1.69 g/cm3 |
| Melting point | 163 °C (325 °F; 436 K) |
| 15 g/100 g | |
| Solubility | soluble in alcohol[1] |
| Hazards | |
| In the name of Allah the most beneficient
the merciful. Some Chemical Equations Introduction Here are some equations: Equations eq. no Equations 2H2+O2→2H2O C+O2→CO2 CuSO4+Fe→FeSO4+Cu AlCl3+Fe→FeCl3+Al CaCl2+Cl2+C→CCl4+Ca 4Na+O2→2Na2O Fe+S→FeS 2Fe+O2→2FeO 2Zn+O2→2ZnO CaO+CO2→CaCO3 H2+I2→2HI H2+F2→2HF H2+Cl2→2HCl H2+Br2→2HBr 2Pb+O2→2PbO SiO2+4Na→Na2O+Si 2H2+C→CH4 N2+3H2→2NH3 2C+H2→C2H2 C2H5OH→H2O+C2H4 CO2+SO2→2O2+C+S Cs2(SO4)3+3H2→3H2SO4+2Cs 2NaOH+H2+Cl2→2NaCl+2H2O H2S+Fe→FeS+H2 CaCl2+2Na→Ca+2NaCl CaSO4+Cu→CuSO4+Ca Reaction of Acid with metal oxide Acids react with metal oxides to form salt and water. There are some of such equations: 27. FeO+H2SO4→FeSO4+H2O 28. ZnO+2HCl→ZnCl2+H2O 29. CuO+2HNO3→H2O+Cu(NO3)2 30. PbO+2HNO3→Pb(NO3)2+H2O 31. Na2O+2HNO3→H2O+2NaNO3 32. FeO+2HNO3→Fe(NO3)2+H2O 33. ZnO+2HNO3→H2O+Zn(NO3)2 34. FeO+2HCl→FeCl2+H2O 35. ZnO+H2SO4→ZnSO4+H2O 36. CuO+2HCl→CuCl2+H2O 37. PbO+2HCl→PbCl2+H2O 38. Na2O+2HCl→2NaCl+H2O 39. CaO+2HCl→CaCl2+H2O 40. MgO+2HCl→MgCl2+H2O 41. CuO+H2SO4→CuSO4 42. PbO+H2SO4→PbSO4+H2O 43. Na2O+H2SO4→Na2SO4+H2O 44. CaO+H2SO4→CaSO4+H2O 45. MgO+H2SO4→MgSO4+H2O Reaction of Acid with metal Acids react with metals to give salt and water. Some chemical equations are given below: 46. H2SO4+Cu→CuSO4+H2 47. H2SO4+Zn→ZnSO4+H2 48. 2HCl+Ca→CaCl2+H2 49. 2HCl+Mg→MgCl2+H2 50. Fe+2HNO3→Fe(NO3)2+H2O Reaction to prepare ozone artfically Scientists tell us that the formula of ozone is O3. This layer protects the Earth from radioactive rays (which are alpha, beta and gamma rays). The equation to prepare ozone artifially: 2O2+O2→2O3 Reaction to estinguish fire In the burning fire process it happens by reaction of oxygen gas (O2) and carbon/sulphur. To lit fire we first have to remove carbon/sulphur. Here is an equation to lit fire: CO2+2H2→2H2O+C Or SO2+2Ca→2CaO+S Redox reactions to remove greenhouse gases Reactions in which oxidation state or oxidation number (a partial charge assigned to a substance) changes are called oxidation-reduction or redox reactions. When rays of the Sun reach the Earth's atmosphere, some of them hit dust particles, and some are reflected by them. Some hit the clouds are absorbed by them. Some gases help the rays that not go back to space. The gases are called greenhouse gases. This whole process (excluding the definition of redox reactions) is called Greenhouse effect. Carbondioxide (CO2), Methane (CH4) and Nitrous oxide (N2O) are some important greenhouse gases. I will tell you how to make these gases useful. Here are equations to make these gases harmless: N2O+2O2+H2O+2CaO→2CaNO3+H2+O2 CH4+C+3O2+CaO→2CaCO3+H2O CO2+H2O+NaOH→H2O+NaHCO3 | |
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa).
| |
Urea nitrate is a fertilizer-based high explosive that has been used in improvised explosive devices in Afghanistan, Pakistan, Iraq, and various other terrorist acts elsewhere in the world, like the 1993 World Trade Center bombings.[2] It has a destructive power similar to better-known ammonium nitrate explosives, with a velocity of detonation between 11,155 ft/s (3,400 m/s) and 15,420 ft/s (4,700 m/s).[3]
Urea nitrate is produced in one step by reaction of urea with nitric acid. This is an exothermic reaction, so steps must be taken to control the temperature.
Urea nitrate explosions may be initiated using a blasting cap.[3]
Chemistry
Urea contains a carbonyl group. The more electronegative oxygen atom pulls electrons away from the carbon forming a greater electron density around the oxygen, giving the oxygen a partial negative charge and forming a polar bond. When nitric acid is presented, it ionizes. A hydrogen cation contributed by the acid is attracted to the oxygen and forms a covalent bond [electrophile H+]. The electronegative NO3− ion then is attracted to the positive hydrogen ion. This forms an ionic bond and hence the compound urea nitrate.
(NH2)2CO (aq) + HNO3 (aq) → (NH2)2COHNO3 (s)
The compound is favored by many amateur explosive enthusiasts as a principal explosive for use in larger charges. In this role it acts as a substitute for ammonium nitrate based explosives. This is due to the ease of acquiring the materials necessary to synthesize it, and its greater sensitivity to initiation compared to ammonium nitrate based explosives.
References
- ^ http://cameochemicals.noaa.gov/chemical/12966
- ^ Aaron Rowe (18 September 2007). "Chem Lab: Spray-On Test for Improvised Explosives". Wired.
- ^ a b "Explosives - ANFO (Ammonium Nitrate - Fuel Oil)". GlobalSecurity.org.
Further reading
- Almog J, Burda G, Shloosh Y, Abramovich-Bar S, Wolf E, Tamiri T (November 2007). "Recovery and detection of urea nitrate in traces". J. Forensic Sci. 52 (6): 1284–90. doi:10.1111/j.1556-4029.2007.00551.x. PMID 17868267.
- Mr.X (July 2008). "Improvised Urea Nitrate". aware eZine Gamma.

