Cyclopentadienyl anion
| |||
| Names | |||
|---|---|---|---|
| IUPAC name
Cyclopentadienide
| |||
| Identifiers | |||
3D model (JSmol)
|
|||
| ChemSpider | |||
PubChem CID
|
|||
CompTox Dashboard (EPA)
|
|||
| |||
| |||
| Properties | |||
| [C 5H 5]− or Cp− | |||
| Molar mass | 65.09 g/mol | ||
| Conjugate acid | Cyclopentadiene | ||
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa).
| |||
In chemistry, the cyclopentadienyl anion or cyclopentadienide is an aromatic species with a formula of [C
5H
5]−
and abbreviated as Cp−.[1] It is formed by the deprotonation of cyclopentadiene. The cyclopentadienyl anion is a ligand which binds to a metal in organometallic chemistry.[2]
Resonance and aromaticity
[edit]The cyclopentadienyl anion is a planar, cyclic, regular-pentagonal ion; it has 6 π-electrons (4n + 2, where n = 1), which fulfills Hückel's rule of aromaticity. Each double bond and lone pair provides 2 π-electrons, which are delocalized into the ring.[3]
Cyclopentadiene has a pKa of about 16. It is acidic relative to many carbon acids. The enhanced acidity is attributed to stabilization of the conjugate base, cyclopentadienyl anion.
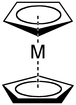
Ligand
[edit]Cyclopentadienyl anions form a variety of cyclopentadienyl complexes.[4]
See also
[edit]- Cyclopentadienyl radical, [C
5H
5]• - Cyclopentadienyl cation, [C
5H
5]+ - Cyclooctatetraenide anion, [C
8H
8]2−
References
[edit]- ^ "Cyclopentadienide". PubChem Compound Database. National Center for Biotechnology Information. Retrieved 14 April 2016.
- ^ Smith, Michael B.; March, Jerry (2007), Advanced Organic Chemistry: Reactions, Mechanisms, and Structure (6th ed.), New York: Wiley-Interscience, p. 66, ISBN 978-0-471-72091-1
- ^ Paul, Satadal; Goswami, Tamal; Misra, Anirban (2015-10-01). "Noncomparative scaling of aromaticity through electron itinerancy". AIP Advances. 5 (10): 107211. doi:10.1063/1.4933191.
- ^ C. Elschenbroich (2006). Organometallics. VCH. ISBN 978-3-527-29390-2.



