- 1College of Geology and Environment, Xi’an University of Science and Technology, Xi’an, China
- 2Institute of Ecological Environment Restoration in Mine Areas of West China, Xi’an University of Science and Technology, Xi’an, China
- 3Shaanxi Provincial Key Laboratory of Geological Support for Coal Green Exploitation, Xi’an, China
Background: Phytogenic mounds are a type of microtopography formed under perennial plants canopies in water erosion areas. However, the function of phytogenic mounds in seed assemblages and their ecological consequences remain poorly understood in semiarid areas with water erosion. Thus, understanding the characteristics of seed banks on mounds is crucial for ecosystem conservation and management in water-eroded areas.
Methods: We compared the quantity and composition of soil seed banks on the upslope and downslope parts of mounds and intercanopy surfaces along four slope gradients. We also explored the relationships among the soil seed bank, aboveground vegetation, and environmental factors. Furthermore, the species similarity between the soil seed bank and aboveground vegetation was analyzed to clarify the important ecological consequences of phytogenic mounds for plant community construction in serious soil erosion area.
Results: For slopes with α ≤ 46.6%, the intercanopy surfaces had greater soil seed bank species composition, density, and diversity than did the phytogenic mounds, and these characteristics showed no significant differences between the upslope and downslope parts of the mounds. As the slope increased, the soil seed bank density and species composition increased on the upslope part of the mound, and reached a maximum for slopes with α > 70%, while the downslope part of the mound negatively effected on seed aggregation. The sediment accumulation rate, soil moisture, particle size distribution, pH, organic matter carbon, and hardness were significantly correlated with the soil seed bank density and diversity in the study area. For slopes with 0 < α ≤ 26.8%, the species similarity coefficient between the soil seed bank and aboveground vegetation was the highest for the intercanopy surface. This species similarity on the upslope part of the mound showed an increasing trend with increasing slope gradient, while the downslope part of the mound had the opposite trend. For slopes with α > 70%, the upslope part of the mound did not only have more species in the soil seed bank but also had more species in aboveground vegetation than did the downslope part of the mound and intercanopy surface.
Conclusion: For slopes with α ≤ 46.6%, phytogenic mounds had barely impact soil seed bank accumulation and conservation in semiarid and eroded areas. For slopes with α > 46.6%, the mounds (particularly on the upslope part of the mound) showed seed assemblage functions, which are coupled with improving edaphic conditions and decreasing microhabitat stress; thus, phytogenic mounds, or areas of microtopography, can be used to promote restoration success in semiarid eroded areas.
1 Introduction
Perennial plants can be physical barriers, accumulating runoff sediments from uphill areas, and their canopy can protect the underlying soil from erosion in arid and semiarid eroded areas. These effects lead to a microtopography called phytogenic mounds (Bochet et al., 2000). These mounds cause spatial heterogeneity in biotic or abiotic resources, which may be important in determining soil biological activity and plant community structure in harsh environments (El-Bana et al., 2007). Thus, understanding the ecological consequences of phytogenic mounds has become a popular topic in semiarid and arid ecosystems (Venier et al., 2023).
Phytogenic mounds, or areas of microtopography, are important in promoting important ecosystem functions in arid and semiarid regions. First, phytogenic mounds can act as “fertility islands” by capturing nutrient-rich surface runoff and sediment eroded from uphill positions (Goudie, 2022); thus, the soil on phytogenic mounds has a higher water content and more nutrients than surrounding interplant spaces (Escudero et al., 2004). This improvement in soil physicochemical properties also enhances soil biological activity, such as high soil enzymatic activity and microbial biomass (Deák et al., 2017). Second, the host plants of mounds can act as “nurse plants,” providing more suitable habitats for understory plant establishment and development (Ba et al., 2022). For example, the host plant canopy can decrease soil temperature (da Silva et al., 2024), and underlying litter can improve water infiltration, which is important for improving microhabitats for flora in semiarid zones (Du et al., 2017). Therefore, phytogenic mounds have generally been regarded as “safe sites” that can create, modify and maintain habitats by improving and conserving soil water availability and accumulating and conserving soil nutrients, thus increasing the productivity and biodiversity of plants in arid and semiarid lands. However, studies have shown that the host plants of mounds may compete with other plants for resources such as water, nutrients, and sunlight, potentially limiting the growth and survival of neighboring plant species, this can negatively impact the diversity and abundance of plant species in the area (Bennett and Klironomos, 2019). Additionally, the continuous accumulation of sediment in mounds negatively impacts plant growth because these processes disturb seedling establishment (Huang et al., 2018). Therefore, the ecological consequences of phytogenic mounds in improving soil properties and maintaining biodiversity need further study.
Phytogenic mounds also represent a “species pool” for many species by altering seed capture processes, especially on eroded land (Melnik et al., 2018). Soil erosion leads to recurrent superficial soil loss and seed removal through runoff or windflow on barren interplant spaces (Bochet et al., 2000). Moreover, mounds and their host plants can restrict seed loss by reducing runoff or wind velocity within or around the canopy, and can trap seeds and propagules carried by runoff or wind from nearby areas (Vulliet et al., 2024). Thus, mounds have greater soil seed banks than adjacent area (Farrell et al., 2012). Moreover, improving soil quality on mounds and enhancing habitats under the host plant canopy may affect seed viability and longevity, which is conducive to long-term conservation and prolonged seed life (Shang et al., 2016). This seed aggregation effect, coupled with rich soil water and nutrient resources, enables the seeds on mounds to germinate and grow efficiently (Braz et al., 2014), which leads to more plant species on mounds than the surrounding areas. Moreover, it seems that mound are important in structuring the plant community through the growth of some keystone plant species, which in turn influence long-term vegetation dynamics and ecosystem processes (Pongen, 2024). On the other hand, research has shown that the host plant canopy can prevent anemophilous seeds from falling on mounds (Bohrer et al., 2008), thereby reducing the seed accumulation on mounds. Therefore, both the positive and negative effects of phytogenic mounds occur simultaneously and shift with environmental conditions, which seems to be more complex than previously assumed. Information regarding seed assemblages on phytogenic mounds or “nebkhas” and their ecological consequences is available mainly for arid desert regions, while there is little evidence about which of these seed trapping affect in semiarid soil erosion areas. The relative importance of trapping seeds and prolonging seed life induced by the mound and its host plants is not clear and requires further investigation.
In a study (Du et al., 2013; Du et al., 2017; Du et al., 2020), we experimentally demonstrated that phytogenic mounds in water erosion areas are formed due to water erosion on bare soil and sediment accumulation under plants; thus, these mounds maintain high soil quality and plant diversity, especially on steep slopes. As a result, we hypothesized that sediment accumulation on mounds is accompanied by seed trapping. However, seed trapping has not been sufficiently researched in water erosion areas. Understanding composition and quantity of seed banks on phytogenic mounds and their influencing factors not only enables us to theoretically predict the ecological resilience of the seed bank, which is an important indicator of ecosystem resilience (Ma et al., 2019) but also enables us to assess the potential of the seed bank for natural restoration processes in eroded areas because the seed bank could represent the local species pool for restoration (Ludewig et al., 2021).
In this paper, we investigated soil seed bank on phytogenic mounds to determine whether they can trap seeds eroded from uphill positions to build a large seed bank. Therefore, the quantity and composition of soil seed banks on the upslope and downslope areas of mounds and intercanopy surfaces at four slope gradients were analyzed. The relationships among aboveground vegetation, soil factors, and the soil seed bank were analyzed to explore the ecological functions of seed bank on phytogenic mound in semiarid areas with water erosion. We addressed the following hypotheses: (I) Seed trapping effects lead to greater density and diversity of the soil seed bank on phytogenic mounds than on intercanopy surfaces, especially on the upslope side of the mound. (II) The spatial heterogeneity of soil sediment accumulation and environmental factors may be vital in determining soil seed bank structure. (III) There is a high level of species composition similarity between the soil seed bank on the mound and the surrounding aboveground vegetation, therefore, phytogenic mounds should have important effects on the natural restoration of soil erosion-disturbed areas.
2 Materials and methods
2.1 Study site description
Five small watersheds with an area of 32 km2 in the Yanhe watershed in northern China (109 15′N, 3644′E) were selected for this study (Figure 1). The study area is a typical loess area with hills and gullies, and the soil type is dominated by loessal soil (FAO). The rainfall is distributed unevenly throughout the year, and the annual precipitation is mainly concentrated in summer. The area has a semiarid climate, with an average annual precipitation of 542.5 mm, an average annual temperature of 8.8°C, an average elevation of 1010–1430 m (average topographic slope of 54%), and an average aridity index of 1.5. The area is characterized as a forest-grassland vegetation zone, and the vegetation consists mostly of Gramineae, Asteraceae, Leguminosae and Rosaceae species (Wang et al., 2020).
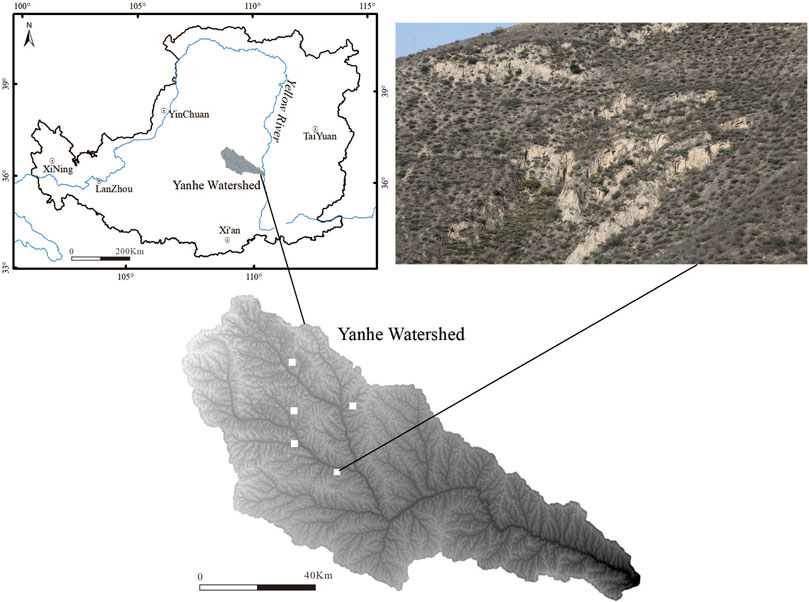
Figure 1. Location of the study area in the Yanhe Watershed, Loess Plateau (top left) and the five small watersheds sampled in this study (filled white squares, bottom left image); the vegetation distribution pattern of the study area is also shown (images taken during sampling).
2.2 Plot selection
The sample plot selected in this study was in a loess gully area, where the vegetation has been naturally restored for 40 years. The sampling plots was arranged on the southern slope where vegetative coverage is approximately 10%–25%. The vegetation was discontinuous, with isolated plants growing in bare soil, and mounds always developed under plant canopies (Figure 2A). The phytogenic mound was approximately 0.15–0.50 m high, with an area of 0.4–1.5 m2. To compare the changes in soil seed bank characteristics on phytogenic mounds across slope gradients, four slope gradient classifications were established according to the Soil Erosion Classification and Gradation Standard set forth by the Ministry of Water Resources of the People’s Republic of China (MWR, 2008), i.e. 0 < α ≤ 26.8%, 26.8 < α ≤ 46.6%, 46.6 < α ≤ 70%, and α > 70%. Fifteen phytogenic mounds were randomly selected and sampled from each slope class.
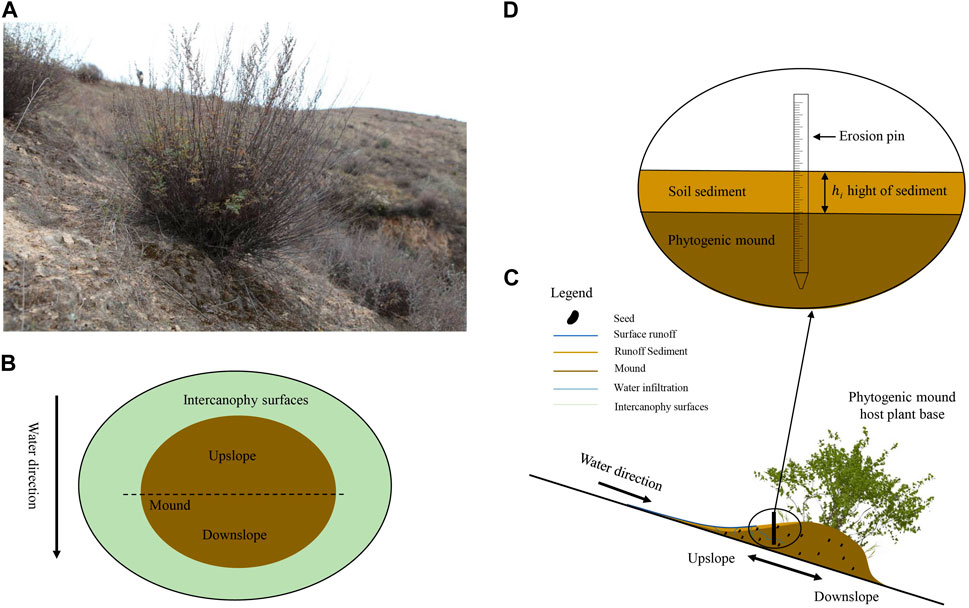
Figure 2. Development of a phytogenic mound under a plant canopy in the Yanhe Watershed, Loess Plateau, China. Phytogenic mound under an isolated plant (A); schematic top view of a mound and the sampling sites (B, C); and erosion pin (D) for measuring sediment accumulation rates.
2.3 Sampling method
2.3.1 Vegetation survey on phytogenic mounds
For each phytogenic mound, two parts were set up based on the slope runoff direction: the upslope and downslope parts (Figure 2B). Before soil seed bank sampling, vegetation surveys were first conducted on each part of the mound and intercanopy surface. The full upslope and downslope parts of the mound were included in the vegetation survey area. The number, coverage, crown width, and height of each plant species were recorded separately. After the vegetation survey, the radius of the base of the mound and the height of the mound were measured to calculate the vegetation survey area (Du et al., 2020).
2.3.2 Soil seed bank sampling method
After the vegetation survey, the soil seed banks on the upslope and downslope parts of the phytogenic mound were sampled. Ten soil cores were collected from each part, and each core had a depth of 10 cm and dimensions of 5 cm in both width and length. They were later mixed to form a single representative soil sample. After manually removing the gravel and incidental debris, the soil samples were placed in fabric bags, transported to the laboratory, and subsequently subjected to an air-drying process.
2.4 Experimental methods
2.4.1 Seed germination
The seedling emergence method was used in this study to determine the species composition and abundance of the soil seed banks (Thompson and Grime, 1979). First, the air-dried soil samples were concentrated following the method of Ter Heerdt et al. (1996). Briefly, soil with particle sizes less than 0.2 mm was screened through a soil sieve to remove fine soil material without seeds. The concentrated samples were spread evenly into germination trays with sterilized sand (115°C–120°C for 48 h), and the soil layer was kept maintained at 2 cm depth. Germination experiments were carried out in a temperature- and light-controlled greenhouse at which the temperature was maintained at 20°C–30°C. Four hundred-watt high-pressure sodium lamps provided growth illumination, with a light regime of 10 h of darkness and 14 h of light. The resulting 180 trays and 5 additional sample-free control trays (to recognize potential airborne seed contamination) were randomly arranged within the greenhouse.
From early April until early October, trays were irrigated daily or every other day, as necessary, to preserve soil moisture. Germinated seedlings were regularly counted and identified, while seedlings that were challenging to identify were transferred to separate containers to promote continued growth until identification became feasible. When there was no seedling emergence within 2 weeks, the soil sample was stirred and persistently observed for germination until plant emergence ceased. When no more seedlings emerged after the second germination period, a gibberellin solution (1 g L−1) was sprayed on the soil samples to prevent seed dormancy. The germination experiment was finished when there was no seedling emergence for 4 weeks.
2.4.2 Analysis of soil physical and chemical properties
To explore the relationships among the soil seed bank, aboveground vegetation, and environmental factors, soil samples were collected at a depth of 0–10 cm on each part of mound and intercanopy surface. The soil hardness was directly assessed from the profile wall using a TF-3 measuring device. The soil bulk density was measured for the soil cores using a cutting ring with dimensions of 50.46 mm in diameter and 50 mm in height (volume:100 cm3). The soil water content was determined through a 24-h drying process at 105°C. The soil samples used for other physical and chemical property analyses were air-dried before analysis. The pH levels were assessed using a pH meter. A Bettersize 2000 E laser particle size analyzer was utilized for particle size determination. Soil organic carbon was measured using the dichromate oxidation method. The above soil properties were analyzed following the methods of Jones (2018).
To measure surface light intensity, a Vantage Pro 2 handheld mini-automated weather station was used. Measurements of sediment accumulation rates were carried out over 1 year, from July 2020 through July 2021. The sediment accumulation rates were recorded using erosion needles laid out on a 5 cm × 5 cm grid on the UP and DN and on the IS (Figures 2C, D). The erosion needles indicated changes in the soil surface level over time. Sedimentation was recorded every year, and the annual rates were calculated as following Eq. 1; (Du et al., 2020):
where SA is the sediment accumulation rate (SA, g cm−2 year−1), n is the number of erosion needles, hi is the increase or decrease in the soil surface height (cm), i is monitoring grid i, A is the upslope or downslope mound area (cm2) and BD is the average soil bulk density (g cm−3).
2.5 Statistical analysis
Seed density was 4 calculated as the average number of emerged seedlings per square meter from the soil samples. Species diversity indices of the soil seed banks were measured based on the following Eqs 2–5 (Begon and Townsend, 2021):
The Margalef index:
Shannon‒Wiener index:
Simpson index:
Pielou index:
where S represents the number of plant species, N represents the total number of individuals of all plant species in one quadrat, and Pi represents the relative abundance of plant species i in one quadrat. Pi was calculated as follows: Pi = ni/N.
The species similarity between the soil seed bank and standing vegetation was calculated using the following Eq. 6 (Hopfensperger, 2007):
where Sc is Sorensen similarity index, a is the number of species in the soil seed bank, b is the number of species in the standing vegetation, and c is the number of species shared by both.
The comparison of plant community attributes on distinctive mound sections with diverse slope gradients was performed using one-way analysis of variance (ANOVA) and least significant difference (LSD) multiple comparisons at a significance level of p < 0.05. These analyses were performed utilizing IBM SPSS statistics software package 24.0. Canoco 5.0 software was used to conduct a redundancy analysis (RDA) of the impact of environmental variables on the plant community composition within the soil seed banks present on the mounds. To explore the similarity between the soil seed banks and aboveground vegetation species, the Sorensen similarity index was calculated using Excel 16.0, and the UpSet R v1.4.0 package was used to visualize the intersecting species between soil seed banks and aboveground vegetation in different sampling plots (Conway, et al., 2017).
3 Results
3.1 Characteristics of the soil seed bank
3.1.1 Density
Overall, the soil seed bank densities on the intercanopy surfaces and downslope part of the mound decreased as the slope increased, but the density in the upslope part of the mound increased with increasing slope (except for slopes with α > 70%). The soil seed bank density on the intercanopy surfaces was 99% greater than that on the mounds with slopes showing 0<α ≤ 26.8% (p < 0.05) (Figure 3), and it was significantly greater on the intercanopy surfaces and upslope part of the mounds than on the downslope part of the mounds at 26.8 < α ≤ 46.6% (p < 0.05). However, the soil seed bank densities on the upslope part of the mound increased by 307% and 152% compared with those on the downslope part of the mound and intercanopy surface for slopes with α > 46.6%, respectively. There was no significant difference in the soil seed bank density between the intercanopy surface and downslope parts of the mound for slopes with α > 46.6% (P < 0.05).
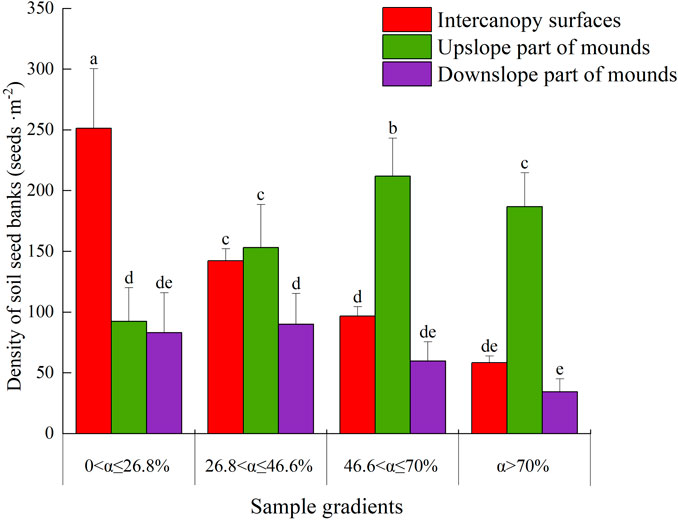
Figure 3. The seed density of the soil seed bank on phytogenic mounds and intercanopy soil surfaces at different slope gradients. The values are denoted as the mean ± SD, and the differences among means were determined by ANOVA and LSD. Different letters denote significant differences on the upslope and downslope parts of the mounds and intercanopy surfaces at different slope gradients (P < 0.05).
3.1.2 Species composition
A total of 44 species were identified from seeds germinated from all soil seed banks collected from the four slope gradients on phytogenic mounds. The top three families were Compositae (11 species), Fabaceae (8 species), and Poaceae (8 species) (Supplementary Table S1). Overall, annual and perennial herbs constituted a large proportion of the seed bank (Figure 4). For slopes with 0 < α ≤ 46.6%, the proportion of annual plants accounted for 52% on average. Moreover, it decreased by 36% and 55% on mounds and intercanopy surfaces, respectively, for slopes with α > 46.6%. Except for 46.6 < α ≤ 70%, the proportion of annual plants did not significantly differ among the parts of the mound for 26.8 < α ≤ 46.6% and α > 46.6%. In contrast to annual plants, the proportion of perennial plants in the seed bank increased with increasing slope gradient and increased from 31% on slopes with α ≤ 46.6%–63% with α > 46.6%. The shrub and tree seeds appeared only on the upslope part of the mound at α > 46.6%, and the proportion was approximately 2%.
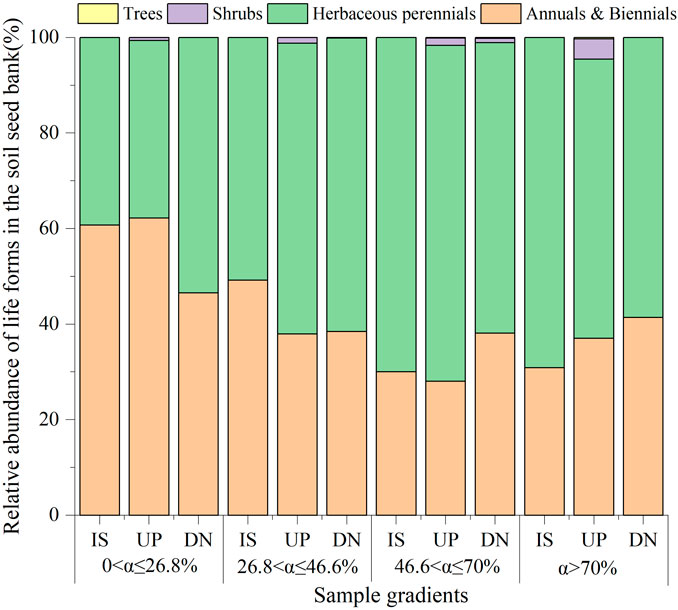
Figure 4. Relative abundance of different growth forms for the soil seed bank on phytogenic mounds and intercanopy soil surfaces at different slope gradients. IS: intercanopy surfaces, UP: upslope part of mounds, DN: downslope part of mounds.
3.1.3 Species diversity
The Margalef richness index, Shannon-Wiener diversity index, Simpson dominance index and Pielou evenness index did not significantly differ among the intercanopy surface and upslope and downslope parts of the mound for slopes with α ≤ 70%, even though the Simpson index did not significantly differ for slopes with α > 70% (Figure 5). At α > 70%, the Margalef index in the upslope part of the mound increased by 41% and 46% (Figure 6A), the Shannon‒Wiener index increased by 25% and 32% (Figure 6B), and the Pielou index decreased by 46% and 55% (Figure 6D), respectively, compared to those on the intercanopy surface and downslope parts of the mound.
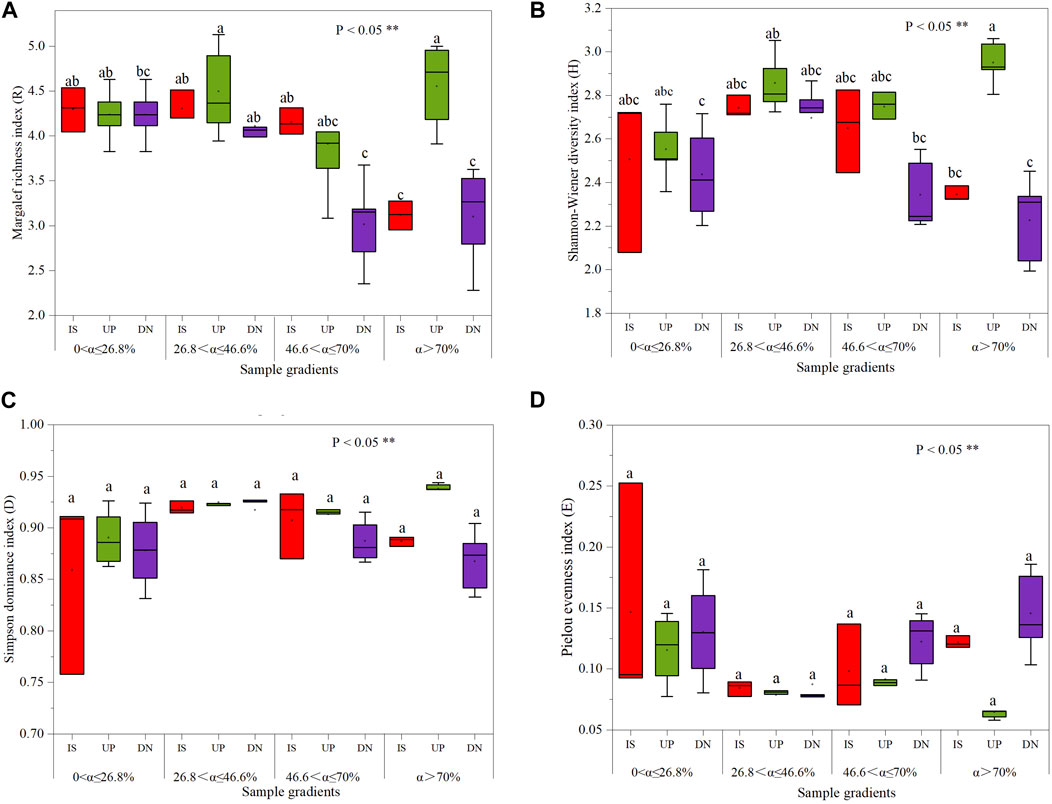
Figure 5. The Margalef index (A), Shannon‒Wiener index (B), Simpson dominance index (C), and Pielou index (D) of soil seed bank on phytogenic mounds and intercanopy soil surfaces at different slope gradients. Two different lowercase letters indicate statistically significant differences concerning the index (significance level P < 0.05). IS: intercanopy surfaces, UP: upslope part of mounds, DN: downslope part of mounds.
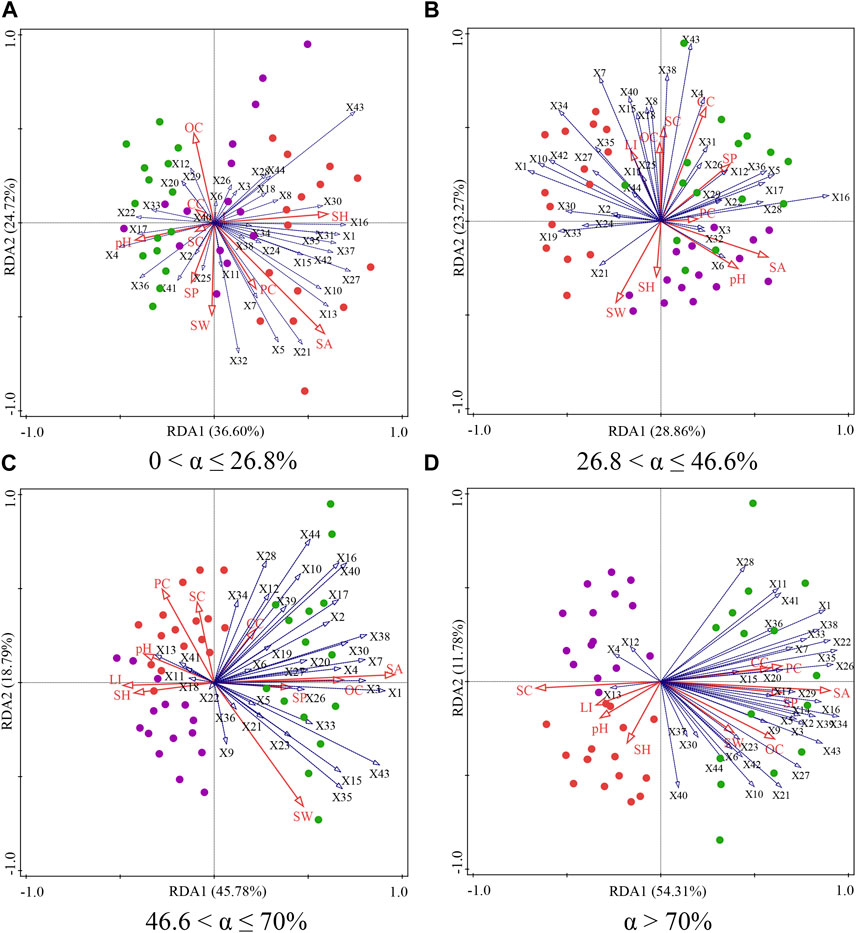
Figure 6. Biplot of the two axes for the RDA (redundancy analysis) for soil factors and soil seed bank on phytogenic mounds and intercanopy soil surfaces at 0 < α ≤ 26.8% (A), 26.8 < α ≤ 46.6% (B), 46.6 < α ≤ 70% (C), and α > 70% (D) slope gradients.



3.2 Relationships between the soil seed bank and soil factors
In general, the sediment accumulation rate, soil water content, particle size distribution were the most critical soil environmental factors affecting the soil seed bank species composition (Table 1). For slopes of 0 < α ≤ 26.8%, the upslope and downslope parts of the mound were clustered on the left side of Axis 1, indicating that there was no difference in the soil physiochemical properties or seed bank species composition on the different parts of the mound. The species richness of the soil seed bank was greater on intercanopy surfaces than on the upslope and downslope parts of the phytogenic mounds, and the species richness was mostly positively correlated with the sediment accumulation rate, soil hardness, and soil water content (Figure 6A). For slopes with 26.8 < α ≤ 46.6%, the species richness was greater on the intercanopy surfaces and the upslope part of the mound. Species richness was positively correlated with the soil clay content, soil particles, soil organic carbon content, and surface illumination intensity (Figure 6B). At α > 46.6%, the species composition of the seed banks had similar distribution patterns according to the RDA ordination diagram (Figures 6C,D). The intercanopy surfaces and downslope part of the mound clustered to the left of Axis 1, and the seed bank species richness was relatively low. The species richness on the upslope part of the mound clustered to the right of Axis 1, where most of the seed bank species were included. Species richness was positively correlated with sediment accumulation rate, soil organic carbon, soil water content, soil clay, and soil porosity but negatively correlated with soil sand, soil bulk density, and soil hardness.
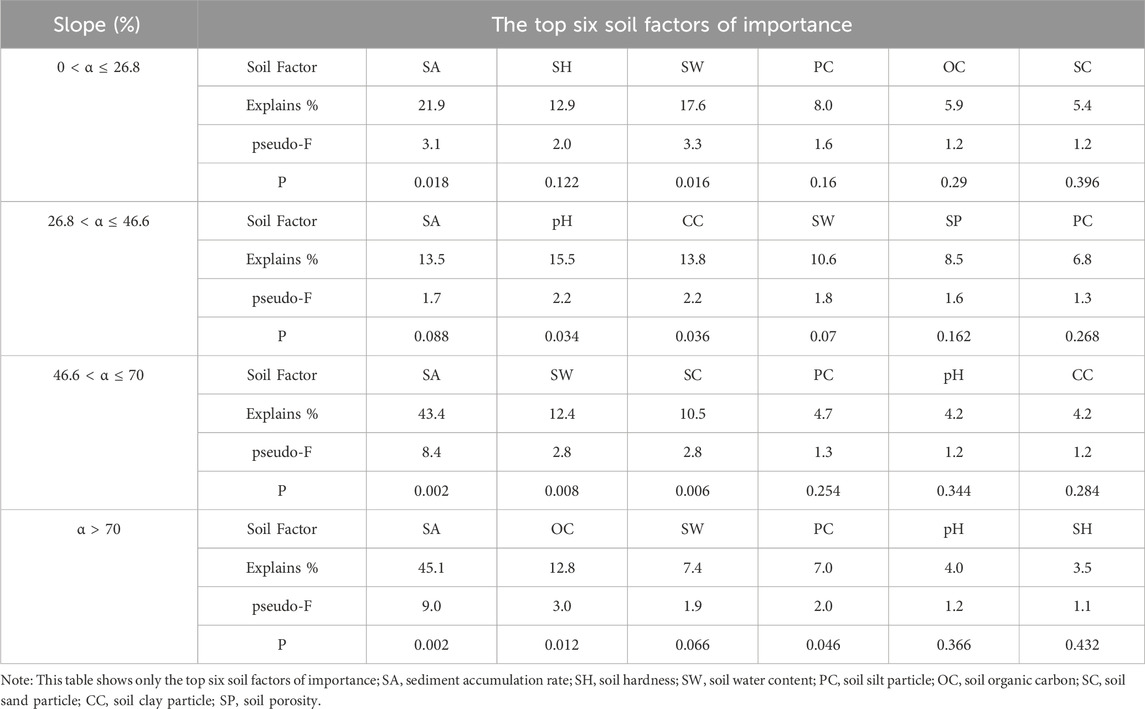
Table 1. Importance and signification level of soil factors affecting the soil seed bank species composition.
3.3 Relationships between the soil seed banks and aboveground vegetation
On the upslope part of the phytogenic mound, the species similarity between the soil seed bank and aboveground vegetation showed an increasing trend with increasing slope, while the downslope part of the mound had the opposite trend. For slopes with 0 < α ≤ 26.8%, the species similarity coefficient between the soil seed bank and aboveground vegetation was the highest on the intercanopy surface, and the species similarity on the mounds decreased by 22% compared with that on the intercanopy surface (Figure 7). Along this slope gradient, there were 5 overlapping species between the soil seed bank and aboveground vegetation, including Potentilla tanacetifolia, Artemisia scoparia, Taraxacum mongolicum, D. moldavica, and Polygala tenuifolia (Figure 8A). For slopes with 26.8 < α ≤ 46.6%, the species similarity between the soil seed bank and aboveground vegetation ranged from 0.41 to 0.47, and there was no difference between the intercanopy surface and different parts of the mound (Figure 7). The overlapping species between the soil seed bank and aboveground vegetation included Stipa bungeana, P. tanacetifolia, D. moldavica, A. scoparia, Melilotus suaveolens, and Poa sphondylodes (6 species) (Figure 8B). For slopes with α > 46.6%, the species similarity between the soil seed bank and aboveground vegetation on the upslope part of the mound increased by 36% and 32%, respectively, compared with that on the downslope part of the mound and intercanopy surface (Figures 7, 8C). For slopes with α > 70%, the upslope part of the mound not only had a greater number of aboveground vegetation species but also had a greater number of specific species in the soil seed bank than did the downslope part of the mound and intercanopy surface, such as the arbor Robinia pseudoacacia; shrubs Ocimum gratissimum, Clematis fruticosa, and Sophora davidii; and herbs Agropyron ciliare and Salsola collina (6 species) (Figure 8D).
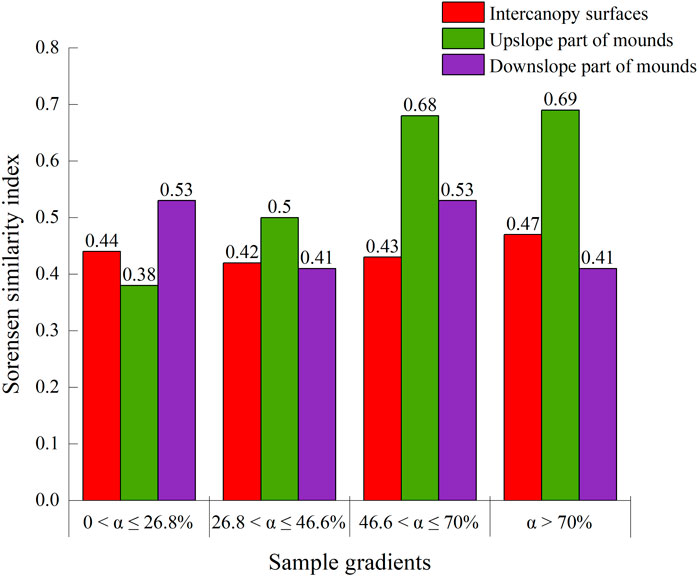
Figure 7. The species similarity matrix of aboveground vegetation and soil seed bank on phytogenic mounds and intercanopy soil surfaces at different slope gradients.
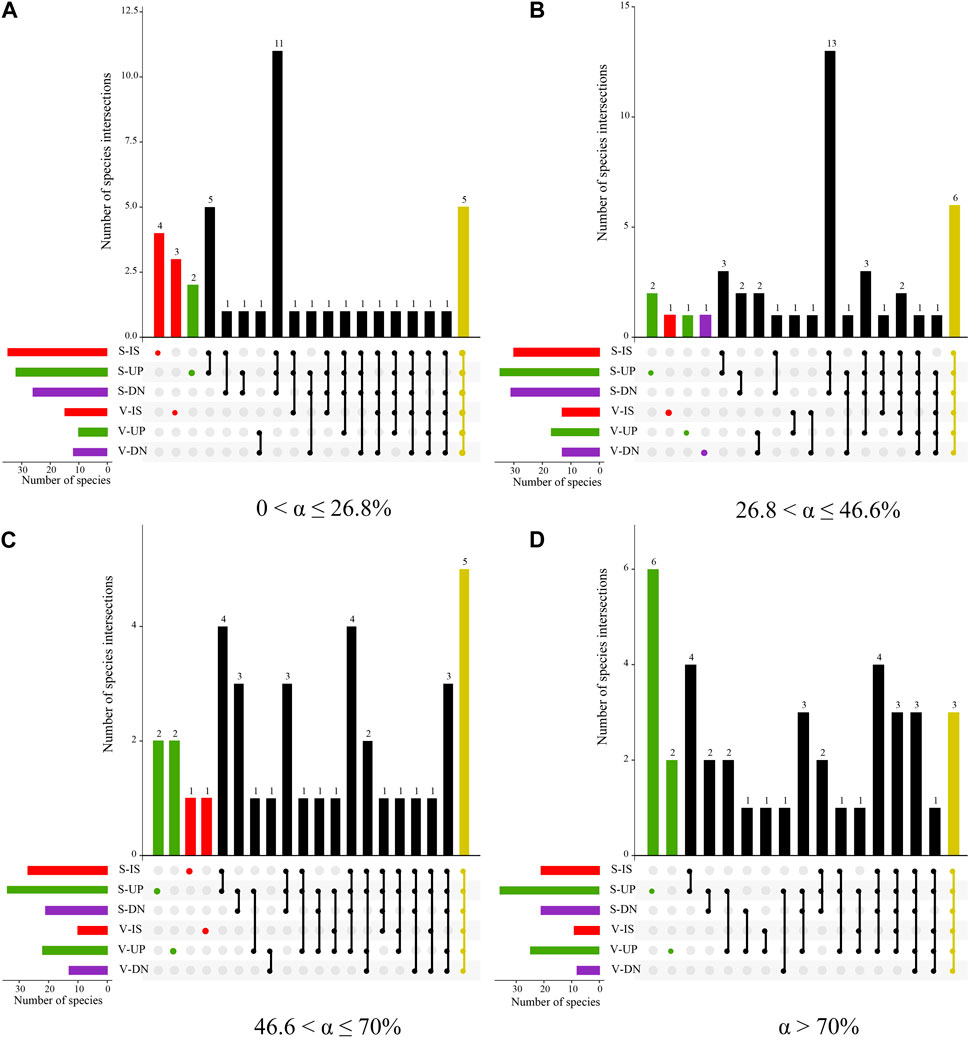
Figure 8. UpSet plot of seedling bank and aboveground vegetation species at 0 < α ≤ 26.8% (A), 26.8 < α ≤ 46.6% (B), 46.6 < α ≤ 70% (C), and α > 70% (D) slope gradients. The horizontal bar on the left represents the number of seedling bank and aboveground vegetation species in sampling plots of different parts of mounds and intercanopy surface. Dots and lines represent two plot associations. Vertical histogram represents the number of intersecting species between these associative plots. Note: I: 0 < α ≤ 26.8%, II: 26.8 < α ≤ 46.6%, III: 46.6 < α ≤ 70%, IV: α > 70%, S, soil seed bank species; V: aboveground vegetation species; IS: intercanopy surfaces, UP: upslope part of mounds, and DN: downslope part of mounds.
4 Discussion
4.1 Accumulation of soil seed banks on phytogenic mounds
Our results indicated that phytogenic mounds exhibit large variations in soil seed bank size across slopes in semiarid and water erosion regions. The soil seed bank density and species number on the phytogenic mounds are significantly lower than those on intercanopy surfaces for slopes with 0 <α ≤ 26.8%, while these soil seed bank characteristics on the upslope part of the mound increase with slope and reach the highest values on slopes with α > 46.4%. This observation confirms Hypotheses I and II in which sediment and seed trapping effects lead to soil seed banks formation on phytogenic mounds. In a previous study, we showed that mounds on gentler slopes are formed due to differences in rain splash erosion beneath plant canopies and surrounding bare surfaces (Du et al., 2013). Thus, only small amounts of sediment and litter that are rich in plant propagules accumulate on the mounds. Moreover, the dense plant canopy blocks windborne seeds from dropping on the mound (Miri et al., 2021). Consequently, mounds seem to negativly effect seed trapping on slopes with α < 46.6%. In contrast, mounds on slopes with α > 46.4% are formed due to sediment accumulation under plants. The topsoil and litter enriched in plant propagules on intercanopy surfaces are removed by slope flow due to the high carrying capacity of surface runoff (Liu et al., 2020), which causes low soil seed bank density and species diversity on slopes with α > 46.6% (Figure 9). The washed material is intercepted by plants and deposited on phytogenic mounds, and the upslope part of the mounds is the main area of seed accumulation (Peralta et al., 2016). This can explain the formation of high soil seed bank density on the upslope part of the mounds. A counterexample of the effects of seed trapping on soil seed bank formation is the characteristics of the seed bank on the downslope part of the mound on slopes with α > 46.6%, which cannot directly block or intercept sediments in runoff from higher slope positions. Thus, the downslope part of mounds with α > 46.6% have the lowest seed bank density and diversity.

Figure 9. Effects of aboveground vegetation and environmental factors on soil seed bank on a phytogenic mound in water erosion area. Note: The red arrow indicates that the external environment positively effects the accumulation and preservation of soil seed banks. The yellow arrow indicates that the external environment does not significantly effect the accumulation and preservation of soil seed banks. Green arrows indicate that the external environment negatively impacts the accumulation and preservation of soil seed banks.
Analysis of the seed bank composition revealed that annual plants predominate in the soil seed bank on intercanopy surfaces and on mounds with different slopes. This is consistent with several studies conducted on the Loess Plateau of China and those conducted in other regions of the world (Rago et al., 2020). Annual plants always produce many seeds, which enables annual plants to quickly colonize disturbed or vacant areas by seed rain or slope runoff, and their seeds can accumulate in the soil over time. In this study, the arbor and shrub seeds appeared only on the upslope part of the mound on slopes with α > 46.6%. These seeds, such as Sophora viciifolia, R. pseudoacacia, and C. fruticosa, commonly have relatively large masses (20–40 mg per seed) and nearly circular shapes (Wang et al., 2011). These seed masses resist water erosion and seed removal processes on slopes with α < 46.6%. However, the transporting power of runoff can move large, round seeds on steep slopes (Janeau et al., 2022), and the mounds intercept slope runoff and intercept these seeds. Moreover, larger seeds are less likely to be incorporated into the soil because they have a lower chance of passively entering through cracks and are more vulnerable to environmental determinants on intercanopy surfaces (Bekele et al., 2022). Moreover, the runoff sediments on the upslope part of mounds can bury seeds and prevent their inactivation from the severe external environment in arid zones. Moreover, mound host plants are nurse plants, reducing soil water evaporation, decreasing thermal stress (Pugnaire et al., 2004), and improving soil quality under the plant canopy, which reduces damage to seed activity from the external environment on steep slopes (Li et al., 2022). This suitable habitat is important for shrubs and trees, which otherwise may fail to establish in more hostile environments (Varela et al., 2021). The emergence of trees and shrubs is not only essential in maintaining species diversity on steep slopes in semiarid areas but also benefits the positive succession of plant communities on steep slopes because trees and shrubs are considered late-stage species in plant community succession (Kalacska et al., 2004). Therefore, our results reaffirmed that phytogenic mounds not only promote seed bank density and diversity but are also refuges for seeds, which can determine plant community dynamics during natural vegetation restoration in areas disturbed by soil erosion (Figure 9).
4.2 Relationships between soil seed banks, soil factors and aboveground vegetation
Contrary to Hypothesis III, our results showed that the species occurring in the seed banks on the mounds had low similarity to the aboveground vegetation except for those on the upslope part of the mounds with slopes at α > 46.6%. These results were consistent with studies on perennial grasslands showing a low correspondence between the species composition of the seed bank and aboveground vegetation (Birhanu et al., 2022). The main reason for this pattern in this research may be that the density of the mound host plant canopy hinders the settlement of seeds on mounds, while the open area of the intercanopy surface can accept seeds from the surrounding area and from distant regions or ecosystems (Witkowski and Garner, 2000). These seeds are also not easily carried away by slope runoff on slopes with α < 46.6%. Another reason for the low level of species similarity between aboveground vegetation and the soil seed bank is that the soil seed bank contains seeds that disappear from aboveground vegetation early in succession and that can survive underground according to their opportunistic species strategy (Amiaud and Touzard, 2004), such as Linum usitatissimum, S. collina, Stenosolenium saxatile, and Androsace umbellate, in this study. However, analysis indicated that the similarity of the seed bank and aboveground vegetation is greater on the upslope part of the mound on slopes with α > 46.6%. This strong similarity between vegetation and the seed bank is attributed to the interception of seed-rich runoff sediments and litter at this site, which, coupled with improving soil physicochemical properties on the upslope part of the mound, may be beneficial for seed viability (Leicht-Young et al., 2009; Wang et al., 2023). Our results indicated that the species composition of the soil seed bank is positively correlated with the soil sediment accumulation rate, soil water content, and particle size distribution. These results are different from those in humid areas where excessively moist soil may reduce seed respiration leading to more seed rot or deterioration (Ma et al., 2017; Zhong et al., 2019), and indicate that good soil aeration and appropriate soil moisture are beneficial for soil seed banks conservation in semiarid areas. In contrast, the intercanopy surfaces and downslope parts of mounds on steep slopes are exposed to strong surface light intensity and excessively dry soil, which potentially accelerate seed inactivation (Figure 9).
The seed bank is generally considered a crucial parameter for plant communities community regeneration in ecologically disturbed areas (Dölle and Schmidt, 2009). Our resluts demonstrated that phytogenic mounds barely impact soil seed bank accumulation and seed bank activity on slopes with α < 46.6%, where plant habitats are less disturbed by soil erosion. The mounds (particularly on the upslope part) maintain greater soil seed bank density and species diversity than do the intercanopy surface on slopes with α > 46.6%, where severe soil erosion interference makes it difficult to renew vegetation (Zuazo and Pleguezuelo, 2009). In this situation, the soil seed bank on the upslope part of the mound serves as a “species pool” for plant propagules accumulation, which provides the necessary foundation for future vegetation restoration and renewal. However, it appears that this promoting effect of plant establishment and growth needs to be accompanied by improved edaphic conditions and decreased microhabitat stress, as in the upslope part of phytogenic mounds. The number and diversity of grass and shrub species established on mounds are evidence that the upslope part of phytogenic mounds on slopes with α > 46.6% is important for natural vegetation restoration in ecosystems degraded by erosion. These results showed that small microtopography, such as phytogenic mounds, can concentrate nutrients and seed resources, which increase seed survival and plant establishment in in severely disturbance areas. Thus, mounds, or areas of microtopography, can be used to promote restoration success in arid and semiarid eroded areas.
5 Conclusion
(1) Phytogenic mounds for slopes with α < 46.6% barely impact soil seed bank accumulation in semiarid and eroded areas, while mounds (particularly those on the upslope part) intercept and preserve plant propagules, leading to high soil seed bank density and species diversity compared with those on intercanopy surfaces for slopes with α > 46.6%.
(2) The sediment accumulation rate, soil water content, and particle size distribution have been suggested as crucial determinants of soil seed bank density and species composition in semiarid regions.
(3) Phytogenic mounds, or small areas of microtopography, can trap runoff, nutrients, and plant seeds. Such simultaneous improvements in abiotic and biotic resources create different habitats that promote the recruitment of species with different growth forms on the mound, which is not only essential in maintaining species diversity in semiarid areas but also benefits the positive succession of plant communities in environments disturbed by severe soil erosion.
Data availability statement
The original contributions presented in the study are included in the article/Supplementary Material, further inquiries can be directed to the corresponding author.
Author contributions
NWJ: Conceptualization, Investigation, Methodology, Writing–original draft, Writing–review and editing. DHD: Formal Analysis, Investigation, Methodology, Resources, Writing–original draft, Writing–review and editing. XSS: Data curation, Investigation, Methodology, Writing–original draft, Writing–review and editing. BYL: Conceptualization, Funding acquisition, Supervision, Writing–review and editing.
Finanzierung
The author(s) declare that financial support was received for the research, authorship, and/or publication of this article. This work was supported by Shaanxi Natural Science Foundation Program [2024JC-YBMS-234]; National key research and development program [2022YFF1303303], National Natural Science Foundation of China [41401306], and Scientific Research Foundation for Doctor, Xi’an University of Science and Technology [8150124007].
Acknowledgments
Acknowledge the assistance of the Shaanxi Provincial Key Laboratory of Geological Support for Coal Green Exploitation and the Institute of Ecological Environment Restoration in Mine Areas of West China.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Supplementary material
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fenvs.2024.1427928/full#supplementary-material
References
Amiaud, B., and Touzard, B. (2004). The relationships between soil seed bank, aboveground vegetation and disturbances in old embanked marshlands of Western France. Flora 199, 25–35. doi:10.1078/0367-2530-00129
Ba, Z. D., Du, H. S., and Wang, S. H. (2022). A review of research on the development and evolution of scrub dunes. Appl. Ecol. Environ. Res. 20, 2343–2363. doi:10.15666/aeer/2003_23432363
Begon, M., and Townsend, C. R. (2021). Ecology: from individuals to ecosystems. New Jersey, USA: John Wiley and Sons.
Bekele, M., Demissew, S., Bekele, T., and Woldeyes, F. (2022). Soil seed bank distribution and restoration potential in the vegetation of Buska Mountain range, Hamar district, southwestern Ethiopia. Heliyon 8, e11244. doi:10.1016/j.heliyon.2022.e11244
Bennett, J. A., and Klironomos, J. (2019). Mechanisms of plant–soil feedback: interactions among biotic and abiotic drivers. New Phytol. 222, 91–96. doi:10.1111/nph.15603
Birhanu, L., Bekele, T., Tesfaw, B., and Demissew, S. (2022). Soil seed bank composition and aboveground vegetation in dry Afromontane forest patches of Northwestern Ethiopia. Trees, For. People 9, 100292. doi:10.1016/j.tfp.2022.100292
Bochet, E., Poesen, J., and Rubio, J. L. (2000). Mound development as an interaction of individual plants with soil, water erosion and sedimentation processes on slopes. Earth Surf. Process. Landforms 25, 847–867. doi:10.1002/1096-9837(200008)25:8<847::AID-ESP103>3.0.CO;2-Q
Bohrer, G., Katul, G. G., Nathan, R., Walko, R. L., and Avissar, R. (2008). Effects of canopy heterogeneity, seed abscission and inertia on wind-driven dispersal kernels of tree seeds. J. Ecol. 96, 569–580. doi:10.1111/j.1365-2745.2008.01368.x
Braz, M. I. G., Rodin, P., and de Mattos, E. A. (2014). Soil seed bank in a patchy vegetation of coastal sandy plains in southeastern B razil. Plant Species Biol. 29, e40–e47. doi:10.1111/1442-1984.12033
Conway, J. R., Lex, A., and Gehlenborg, N. (2017). UpSetR: an R package for the visualization of intersecting sets and their properties. Bioinformatics 33, 2938–2940. doi:10.1093/bioinforma10.1093/bioinformatics/btx364tics/btx364
da Silva, I. A., Merritt, D. J., Erickson, T. E., Mayfield, M. M., and Dwyer, J. M. (2024). Annual species' experimental germination responses to light and temperature do not correspond with their microhabitat associations in the field. J. Veg. Sci. 35, e13252. doi:10.1111/jvs.13252
Deák, B., Tölgyesi, C., Kelemen, A., Bátori, Z., Gallé, R., Bragina, T. M., et al. (2017). The effects of micro-habitats and grazing intensity on the vegetation of burial mounds in the Kazakh steppes. Plant Ecol. Divers. 10, 509–520. doi:10.1080/17550874.2018.1430871
Dölle, M., and Schmidt, W. (2009). The relationship between soil seed bank, above-ground vegetation and disturbance intensity on old-field successional permanent plots. Appl. Veg. Sci. 12, 415–428. doi:10.1111/j.1654-109X.2009.01036.x
Du, H. D., Jiao, J. Y., Jia, Y. F., Wang, N., and Wang, D. L. (2013). Phytogenic mounds of four typical shoot architecture species at different slope gradients on the Loess Plateau of China. Geomorphology 193, 57–64. doi:10.1016/j.geomorph.2013.04.002
Du, H. D., Jiao, J. Y., and Zhao, X. G. (2017). Significance and pedogenic variability of phytogenic mounds on the Loess Plateau of China. J. Arid Environ. 146, 53–63. doi:10.1016/j.jaridenv.2017.06.003
Du, H. D., Ning, B. Y., Jiao, J. Y., and Cao, Y. C. (2020). Spatial heterogeneity of plant community composition and diversity on phytogenic mounds caused by water erosion. Plant Ecol. Divers. 13, 425–436. doi:10.1080/17550874.2021.1897700
El-Bana, M. I., Li, Z. Q., and Nijs, I. (2007). Role of host identity in effects of phytogenic mounds on plant assemblages and species richness on coastal arid dunes. J. Veg. Sci. 18, 635–644. doi:10.1111/j.1654-1103.2007.tb02577.x
Escudero, A., Giménez-Benavides, L., Iriondo, J. M., and Rubio, A. (2004). Patch dynamics and islands of fertility in a high mountain Mediterranean community. Arct. Antarct. Alp. Res. 36, 518–527. doi:10.1657/1523-0430(2004)036[0518:PDAIOF]2.0.CO;2
Farrell, C., Hobbs, R. J., and Colmer, T. D. (2012). Microsite and litter cover effects on seed banks vary with seed size and dispersal mechanisms: implications for revegetation of degraded saline land. Plant Ecol. 213, 1145–1155. doi:10.1007/s11258-012-0072-y
Goudie, A. S. (2022). Nebkhas: an essay in aeolian biogeomorphology. Aeolian Res. 54, 100772. doi:10.1016/j.aeolia.2022.100772
Hopfensperger, K. N. (2007). A review of similarity between seed bank and standing vegetation across ecosystems. Oikos 116, 1438–1448. doi:10.1111/j.0030-1299.2007.15818.x
Huang, T., Zhang, H., Dai, L., Cong, X., and Ma, S. (2018). Formation of banded vegetation patterns resulted from interactions between sediment deposition and vegetation growth. Comptes Rendus. Biol. 341, 167–181. doi:10.1016/j.crvi.2018.01.008
Janeau, J. L., Intanon, S., Pansak, W., Rodprai, C., Anusorn, K., Hammecker, C., et al. (2022). Slope position and biochar influence soil properties and seed displacement in a tropical agroecosystem. Eur. J. Soil Sci. 73, e13216. doi:10.1111/ejss.13216
Jones, J. (2018). Soil analysis handbook of reference methods. Florida, USA: CRC Press. doi:10.1201/9780203739433
Kalacska, M., Sanchez-Azofeifa, G. A., Calvo-Alvarado, J. C., Quesada, M., Rivard, B., and Janzen, D. H. (2004). Species composition, similarity and diversity in three successional stages of a seasonally dry tropical forest. For. Ecol. Manag. 200, 227–247. doi:10.1016/j.foreco.2004.07.001
Leicht-Young, S. A., Pavlovic, N. B., Grundel, R., and Frohnapple, K. J. (2009). A comparison of seed banks across a sand dune successional gradient at Lake Michigan dunes (Indiana, USA). Plant Ecol. 202, 299–308. doi:10.1007/s11258-008-9484-0
Li, M., Xiao, H., Xin, Z., Li, X., Li, J., Miri, A., et al. (2022). Soil seed bank characteristics of Nitraria tangutorum nebkhas in a desert–oasis ecotone. Front. Environ. Sci. 10, 937257. doi:10.3389/fenvs.2022.937257
Liu, J. X., Li, P. P., Liu, G. B., and Flanagan, D. C. (2020). Quantifying the effects of plant litter in the topsoil on the soil detachment process by overland flow in typical grasslands of the Loess Plateau, China. Hydrol. Process. 34, 2076–2087. doi:10.1002/hyp.13713
Ludewig, K., Hansen, W., Klinger, Y. P., Eckstein, R. L., and Otte, A. (2021). Seed bank offers potential for active restoration of mountain meadows. Restor. Ecol. 29, e13311. doi:10.1111/rec.13311
Ma, M., Baskin, C. C., Li, W., Zhao, Y., Zhao, Y., Zhao, L., et al. (2019). Seed banks trigger ecological resilience in subalpine meadows abandoned after arable farming on the Tibetan Plateau. Ecol. Appl. 29, e01959. doi:10.1002/eap.1959
Ma, M., Baskin, C. C., Yu, K., Ma, Z., and Du, G. (2017). Wetland drying indirectly influences plant community and seed bank diversity through soil pH. Ecol. Indic. 80, 186–195. doi:10.1016/j.ecolind.2017.05.027
Melnik, K., Landhäusser, S. M., and Devito, K. (2018). Role of microtopography in the expression of soil propagule banks on reclamation sites. Restor. Ecol. 26, S200–S210. doi:10.1111/rec.12587
Miri, A., Dragovich, D., and Dong, Z. (2021). Wind flow and sediment flux profiles for vegetated surfaces in a wind tunnel and field-scale windbreak. Catena 196, 104836. doi:10.1016/j.catena.2020.104836
MWR (2008). Standard for classification and gradation of soil erosion. Beijing, China: Waterpower Press.
Peralta, A. M. L., Sánchez, A. M., Luzuriaga, A. L., and Escudero, A. (2016). Factors driving species assemblage in Mediterranean soil seed banks: from the large to the fine scale. Ann. Bot. 117, 1221–1228. doi:10.1093/aob/mcw039
Pongen, R. (2024). Keystone species: ecological architects of biodiversity and stability: review. Int. J. Sci. Res. Archive 11, 1137–1152. doi:10.30574/ijsra.2024.11.1.0175
Pugnaire, F. I., Armas, C., and Valladares, F. (2004). Soil as a mediator in plant-plant interactions in a semi-arid community. J. Veg. Sci. 15, 85–92. doi:10.1111/j.1654-1103.2004.tb02240.x
Rago, M. M., Urretavizcaya, M. F., Orellana, I. A., and Defossé, G. E. (2020). Strategies to persist in the community: soil seed bank and above-ground vegetation in Patagonian pine plantations. Appl. Veg. Sci. 23, 254–265. doi:10.1111/avsc.12482
Shang, Z., Yang, S., Wang, Y., Shi, J., Ding, L., and Long, R. (2016). Soil seed bank and its relation with above-ground vegetation along the degraded gradients of alpine meadow. Ecol. Eng. 90, 268–277. doi:10.1016/j.ecoleng.2016.01.067
Ter Heerdt, G. N. J., Verweij, G. L., Bekker, R. M., and Bakker, J. P. (1996). An improved method for seed-bank analysis: seedling emergence after removing the soil by sieving. Funct. Ecol. 10, 144–151. doi:10.2307/2390273
Thompson, K., and Grime, J. P. (1979). Seasonal variation in the seed banks of herbaceous species in ten contrasting habitats. J. Ecol. 67, 893–921. doi:10.2307/2259220
Varela, O., Ordano, M., Toledo, G., Lizardo, G., Rotger, S., Montero, A., et al. (2021). Diversity and density of the desert seed bank: interplays between cacti and nurse shrub species. J. Arid Environ. 191, 104536. doi:10.1016/j.jaridenv.2021.104536
Venier, P., Ferreras, A. E., Lauenstein, D. L., and Funes, G. (2023). Nurse plants and seed provenance in the restoration of dry Chaco forests of central Argentina. For. Ecol. Manag. 529, 120638. doi:10.1016/j.foreco.2022.120638
Vulliet, C., Koci, J., Sheaves, M., and Waltham, N. (2024). Linking tidal wetland vegetation mosaics to micro-topography and hydroperiod in a tropical estuary. Mar. Environ. Res. 197, 106485. doi:10.1016/j.marenvres.2024.106485
Wang, N., He, X., Zhao, F., Wang, D., and Jiao, J. Y. (2020). Soil seed bank in different vegetation types in the Loess Plateau region and its role in vegetation restoration. Restor. Ecol. 28, A5–A12. doi:10.1111/rec.13169
Wang, N., Jiao, J. Y., Jia, Y. F., and Zhang, X. A. (2011). Soil seed bank composition and distribution on eroded slopes in the hill-gully Loess Plateau region (China): influence on natural vegetation colonization. Earth Surf. Process. Landforms 36, 1825–1835. doi:10.1002/esp.2209
Wang, Y., Wang, Y., Wang, S., Wang, M., and Chai, W. (2023). Assessment of topsoil removal as an effective method for vegetation restoration in farmed peatlands. Front. Environ. Sci. 10, 1110057. doi:10.3389/fenvs.2022.1110057
Witkowski, E. T. F., and Garner, R. D. (2000). Spatial distribution of soil seed banks of three African savanna woody species at two contrasting sites. Plant Ecol. 149, 91–106. doi:10.1023/A:1009850706843
Zhong, M., Miao, Y., Han, S., and Wang, D. (2019). Nitrogen addition decreases seed germination in a temperate steppe. Ecol. Evol. 9, 8441–8449. doi:10.1002/ece3.5151
Keywords: soil seed bank, phytogenic mound, vegetation restoration, sediment accumulation rate, water erosion, microtopography
Citation: Nie WJ, Du HD, Xie SS and Bi YL (2024) The function of phytogenic mounds in the accumulation and conservation of soil seed banks in semiarid areas with water erosion. Front. Environ. Sci. 12:1427928. doi: 10.3389/fenvs.2024.1427928
Received: 05 May 2024; Accepted: 12 July 2024;
Published: 05 August 2024.
Edited by:
Qiang Li, University of Houston–Downtown, United StatesCopyright © 2024 Nie, Du, Xie and Bi. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: H. D. Du, [email protected]
 W. J. Nie
W. J. Nie H. D. Du
H. D. Du S. S. Xie1
S. S. Xie1 Y. L. Bi
Y. L. Bi