A Bionic Eye That Restores Sight
By bridging the gap between eye and brain, a new device has the capacity to help the blind regain their vision.
Scientifically speaking, eyes are not the windows to the soul; they’re the windows to the brain. When you gaze into your lover’s peepers, what you’re actually seeing is the retina, an extension of brain tissue that lines the back of the eyes like wallpaper. This paper-thin strip of cells is what makes the miracle of sight possible. At this very moment, your retina is performing a kind of sensory alchemy, taking in rays of light and seamlessly transforming them into the language of the brain. And voila: vision.
What happens when this key conversion doesn’t take place? Lisa Kulik found out the answer in 1981, when she went in for a routine eye exam. Kulik had been having a little trouble seeing at night—nothing she was too concerned about. Then her eye doctor found dark spots on her retina.
Kulik had retinitis pigmentosa, a degenerative eye disease that affects 1 in every 4,000 Americans, according to the National Eye Institute. In retinitis pigmentosa, almost all of the eye’s circuitry remains intact—all but the crucial, light-absorbing cells of the retina. These slowly begin dying off, like stars winking out into the night. Without them, visual signals never make it to the brain.
Over the next 15 years, Kulik’s vision gradually deteriorated. She had to give up her job as a veterinarian’s assistant, forfeit her driver’s license, and finally, retire completely. Yet even after her world went fully dark, Kulik, now 54, remained hopeful. “When they diagnosed me, they told me there was no cure for it,” she says now. “That didn’t stop me. I knew someday, something was going to come along.”
In 2012, something did. Kulik’s husband was scanning news on his smartphone and came across something that sounded too good to be true: a report describing a medical device that promised to restore sight to those with Kulik’s disease. It was called the Argus II. He found the phone number for the company that was developing the device, called Second Sight.
Kulik called the number the next day, and was told that she would receive a call back when the Argus II gained FDA approval. “It was the first light of hope that something could help,” she says.
* * *
For centuries, scientists have marveled at the eye’s seemingly inexplicable complexity. Charles Darwin wrote in The Origin of Species: “To suppose that the eye with all its inimitable contrivances for adjusting the focus to different distances, for admitting different amounts of light, and for the correction of spherical and chromatic aberration, could have been formed by natural selection, seems, I freely confess, absurd in the highest degree.” (Still, he went on to postulate just such an explanation: that a very simple eye first evolved and, through natural selection, progressed in gradations.)
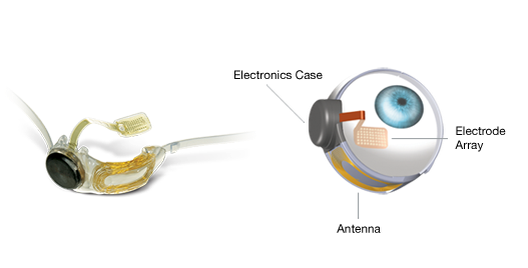
The Argus II works not by seeking to replicate that complexity, but by tapping into the eye’s natural abilities. It bridges the gap between visual signal and brain—or, as Mark Humayun, the Argus’ creator and an opthamologist at the University of Southern California’s Keck School of Medicine, puts it: “The software speaks in the biological language.” The device relies on a small video camera affixed to a pair of sunglasses, which sends visual data to an electrode-covered microchip implanted at the back of the eye. The electrodes stand in for the damaged retinal cells, transmitting electrical signals straight to the optic nerve. The user receives the information in the form of a 60-pixel, black-and-white image.

(Keck School of Medicine)
Humayun likes to compare the Argus II to VISOR, a fictional device in Star Trek: Next Generation worn by Geordi La Forge that similarly scans the electromagnetic spectrum and sends signals to the optic nerve. In fact, Humayun had come up with the idea for the Argus 10 years before the show aired, motivated by watching his grandmother lose her vision due to complications related to diabetes. “There wasn’t anything that could be done,” he says. “It made me reconsider my path in medicine.”
At the time, his design sounded far-fetched. The closest analog was the cochlear implant, which similarly converts sound into electrical impulses that the brain can understand. But Humayun’s undertaking had its own challenges. First, complexity: the ear has 30,000 hair cells, whereas the eye has 1 million ganglion cells, which translate light into pixels. Secondly, it would require implanting an electronic device into the most delicate part of the eye, the retina, where it would be subject to dislodging during the rapid eye movement part of sleep.
Twenty years and $200 million from private and public investors later, the Argus II became the first FDA-approved visual prosthesis available for commercial implant in February of last year. Nearly 80 people have had it implanted worldwide.
In early 2013, Kulik got a call back from Second Sight. She would be the third commercial patient in the U.S., and the first on the West Coast.
* * *
Last July, Kulik flew from Arizona down to USC for a series of eye tests. In the end, Kulik was chosen not only because she fit the requirements—her blindness had to be severe enough that she could benefit from the device—but also because of her optimism and dedication to learning how to use the Argus II, said Lisa Olmos de Koo, the eye surgeon at USC who performed the procedure.
It took nearly a year for Kulik’s private insurance to agree to cover the $150,000 device. Finally, in June of this year, Kulik went under the knife.
First, Olmos de Koo peeled back the outermost layer of the eye, the conjunctiva. Next she wrapped a silicon belt around the eye’s circumference, behind the eye muscles. To reach the back of the eye, she broke up the vitreous, the jelly-like substance that fills the eye, suctioned it out and replaced it with a saline solution. Then came the hard part: she had to place the electrode chip so that it fell squarely into the center of the retina. “You don’t get another chance,” Olmos de Koo says. She tacked the chip in place and hoped for the best.
A few days later, Kulik turned on the device. At first she could only see dramatic contrast: the edges of sidewalks, the steak on her plate at dinner (she still can’t make out rice). “A lot of people think I’m going to put it on and ‘Wow, you’re going to see again.’ It’s nothing like that,” she says. “The contrast is easy, but trying to figure out shapes and letters—I need to work on that more. It’s definitely a whole new way of learning how to see.”
* * *
Humayun dreams of more than just 60 pixels. He wants to fine-tune the resolution of the device so that it can be used for those with macular degeneration, a far more common cause of blindness that affects more than 2 million Americans, according to Prevent Blindness America. That challenge will be “more than just going from two to eight megapixels,” he says. “It’s like going from a train to a plane.”
Yet no matter how much Humayun improves it, there are some things the Argus II will never achieve. For instance, color. That would require wiring each electrode to one of the eye’s matching colored cones—an impossible feat. And the device still requires a user to have an intact optic nerve and other structures of the eye to convey its messages to the brain, a caveat that rules out many other forms of blindness.
In other words, the very brilliance that makes the Argus II possible is also its limitation. The device is a “good stop-gap,” Robert Greenberg, founder and CEO of Second Sight, said in a 2013 interview. But “with these implants, we’re not fixing the disease, we’re bypassing the damaged part.”
Some put it more bluntly. “Right now, we’re just poking the retina,” says Dr. Theodore Leng, an eye surgeon at Stanford University who specializes in retinal surgery. To truly restore a natural sense of sight, Leng says, we will have to break the neural code, the way light is processed to become an image in the brain. If scientists could do that, they could skip the eye entirely and stimulate the brain directly to produce sight. “We’re only scratching the surface as far as trying to replicate visual function,” Leng says.
But between increasing the resolution and breaking neural code, it may be possible for those who were once blind to see faces, landscapes, animals and other objects in “the realm of normal image representation,” Sheila Nirenberg, a neuroscientist at Cornell University, wrote in a 2012 paper.
“Understanding the code is really, really important,” Nirenberg said in a 2011 TED Talk. “If we can understand the code, the language of the brain, things become possible that didn’t seem obviously possible before.”
Still, for some, 60 pixels is enough. This July 4, Kulik saw fireworks for the first time in more than a decade. Of course, they weren’t the same for her as they were for you or I; all she saw was thick and thin flashes of light against a black sky, “like dash-dash-dash,” as she puts it. “I knew what it was because it was flashing like crazy when I looked up.” But she wouldn’t soon forget the experience. “It was very, very exciting,” she says.
Kulik says that what she looks forward to most is regaining her sense of independence. “I know I’ll never drive again, but [I’ll] at least to get around to take a walk by myself, to get around by myself,” she says. And now, she can see her grandchildren.


