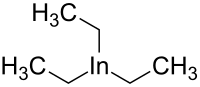
Triethylindium
Appearance
| |
| Names | |
|---|---|
| IUPAC name
Triethylindium
| |
| Other names
Indium triethyl, triethylindigane, indiumtriethyl, TEI, TEIn
| |
| Identifiers | |
3D model (JSmol)
|
|
| ChemSpider | |
| ECHA InfoCard | 100.011.905 |
PubChem CID
|
|
CompTox Dashboard (EPA)
|
|
| |
| |
| Properties | |
| C6H15In | |
| Molar mass | 202.004 g·mol−1 |
| Appearance | Colorless liquid |
| Boiling point | 144 °C (291 °F; 417 K) |
| Hazards | |
| GHS labelling: | |
 
| |
| Danger | |
| H250, H314 | |
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa).
| |
Triethylindium is an
organometallic compound. Its chemical formula is C
6H
15In.[1][2]
Synthesis
[edit]This compound can be obtained by reacting indium(III) bromide with a diethyl ether solution of Ethylmagnesium bromide:
- InBr
3 + 3 C
2H
5MgBr → In(C2H5)3 + 3 MgBr
2
Other routes are also known.[3]
Properties
[edit]Indium triethyl is a colorless, toxic, oxidation and hydrolysis-sensitive liquid. It is a monomer in the gaseous and dissolved state. The compound reacts with halomethanes to form diethyl indium halides.[4]
Triethylindium is highly reactive with water:
- In(C2H5)3 + H
2O → In(C2H5)2OH + C
2H
6↑
Applications
[edit]Indium triethyl is used to prepare indium phosphide layers for microelectronics.[5]
See also
[edit]References
[edit]- ^ "INDIUM TRIETHYL". chemicalbook.com. Retrieved 7 June 2017.
- ^ "Substance Name: Indium, triethyl". chem.nlm.nih.gov. Retrieved 7 June 2017.
- ^ Foster, Douglas F.; Cole-Hamilton, David J. (1997). "Electronic Grade Alkyls of Group 12 and 13 Elements". Inorganic Syntheses. Vol. 31. p. 29 66. doi:10.1002/9780470132623.ch7. ISBN 978-0-471-15288-0.
- ^ Maeda, Takayoshi; Tada, Hisashi; Yasuda, Kiyoshi; Okawara, Rokuro (11 September 1970). "Reactions of triethylindium with halomethanes: preparations and properties of diethylindium halides". Journal of Organometallic Chemistry. 27 (1): 13–18. doi:10.1016/S0022-328X(00)82987-3.
- ^ Sakaki, H.; Woo, J.C.; Yokoyama, N.; Harayama, Y. (1999). Compound Semiconductors: Proceedings of the Twenty-Fifth International Symposium on Compound Semiconductors held in Nara, Japan, 12-16 October 1998. CRC Press. p. 529. ISBN 978-0-7503-0611-9.
