Key Points
- When a chemical reaction happens, energy is transferred to or from the surroundings.
- When energy is transferred to the surroundings, this is called an exothermic reaction and usually feels hot.
- When energy is taken in from the surroundings, this is called an endothermic reaction and usually feel cold.
Can you think of any everyday reactions that either release energy into the surroundings or absorb it?
Reactions that release energy:
- In a combustion reaction, fuel is burned and reacts with oxygen to release energy.
- Respiration is the chemical change that takes place inside living cells. Glucose and oxygen are used to release energy that organisms need to live.
Reactions that absorb energy:
- Photosynthesis absorbs energy from sunlight, allowing plants to make glucose and oxygen from carbon dioxide and water.

Video
Watch this video about different exothermicA physical change or chemical reaction that transfers energy to the surroundings. and endothermicA physical change or chemical reaction that absorbs energy from the surroundings. reactions.
While you're watching, look out for the different household items which can be used for exothermic and endothermic reactions.
A Bunsen burner flame is produced by burning methane gas. The scientific word for burning is combustion. All combustion reactions give off heat into their surroundings. So they’re described as exothermic reactions.
But there are other exothermic reactions out there that don’t require any burning. Here’s one you can try at home.
If you take some laundry detergent, and then mix in a bit of water, you’ll be able to feel it getting warmer.
If I do the same reaction in an insulated cup, to stop the heat escaping into the surroundings, the thermometer shows the temperature of the surroundings increasing as energy is released.
Not all reactions give off heat like this. In fact, some actually take in or absorb energy from their surroundings, and the reacting substances feel cold. They’re called endothermic reactions.
If you take some citric acid solution and combine it with baking soda, we can see the reactants fizzing away. And the surrounding flask feels cold.
When I put the same reactants in an insulated cup to stop heat being transferred from the surroundings, the temperature drops quickly.
These exothermic and endothermic reactions can be really useful in certain situations. There’s an exothermic reaction in hand warmers - start the reaction and crystals will form to create a nice warm package. And there’s an endothermic reaction in cold packs that help when you’ve twisted your ankle.
What happened to the temperature when citric acid and bicarbonate of soda were mixed?
The temperature decreased. We call this an endothermic reaction.
Exothermic reactions
Exothermic reactions are chemical reactionWhen chemical bonds are broken and made between atoms, so that new substances (compounds or elements) are made. which release energy from the chemicals into the surroundings. This energy is usually released as heat, so the surroundings get hotter. Handwarmers are an example of an exothermic reaction. They release heat into their surroundings.
However, exothermic reactions don't always release heat, sometimes the energy is released as light. For example, glowsticks release light without increasing in temperature.

Example of an exothermic reaction
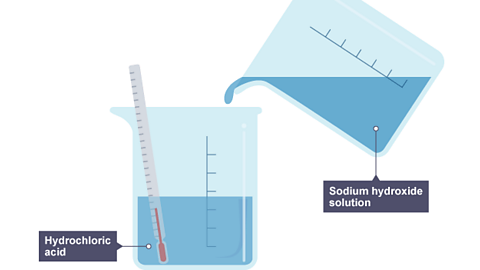
Image caption, 1. Sodium hydroxide solution is poured into a beaker of hydrochloric acid. The thermometer shows the initial temperature, which is room temperature.
Image caption, 2. The beaker now contains sodium chloride and water. The temperature on the thermometer has risen, meaning it is an exothermic reaction.
1 of 2
Respiration is a chemical process that takes place inside the cells of all living organisms. Respiration releases energy for the organism to use to stay alive.
Is respiration an exothermic reaction or an endothermic reaction?
Respiration is an exothermic reaction because it releases energy.
Remember, not all exothermic reactions release energy as heat.
Endothermic reactions

Endothermic reactions absorb energy from the surroundings. This energy is usually absorbed as heat, so the surroundings get colder.
- photosynthesisA chemical process used by plants to make glucose and oxygen from carbon dioxide and water, using light energy. Oxygen is produced as a by-product of photosynthesis. Algae subsumed within plants and some bacteria are also photosynthetic. is an endothermic reaction because plant leaves absorb light energy.
- thermal decompositionBreaking down a compound into its elements, or simpler compounds using a high temperature. reactions are endothermic because they absorb energy when the chemicals are heated.

Example of an endothermic reaction
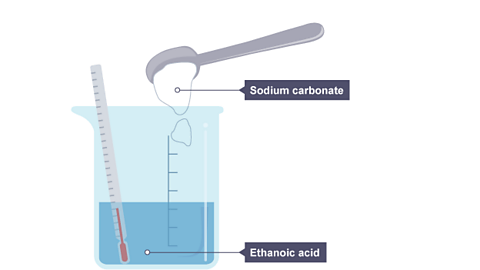
Image caption, 1. Sodium carbonate powder is tipped into a beaker of ethanoic acid. The thermometer shows the initial temperature reading which is room temperature.
Image caption, 2. The beaker now contains sodium ethanoate, water and carbon dioxide. The temperature on the thermometer has fallen, meaning it is an endothermic reaction.
1 of 2
When ammonium chloride powder is mixed with barium hydroxide powder and stirred, a chemical reaction takes place. The temperature drops to –14°C.
True or false? This is an exothermic reaction.
False! It's an endothermic reaction.
This is because the temperature of the surroundings decreased.
Working Scientifically
Exothermic and endothermic reactions that occur at room temperature in the science lab can be investigated using a thermometer.
Here are some things to remember when conducting this type of experiment.
- Conduct the experiment in a polystyrene foam cup. This insulates the mixture and makes the results more accurate.
- Record the temperature of the chemicals before they are mixed together.
- Record the highest or lowest temperature of the mixture after the reaction has occurred.
- If the temperature of the mixture has gone down, the reaction is endothermic.
- If the temperature of the mixture has gone up, the reaction is exothermic.
When investigating exothermic and endothermic reactions, it is important to minimise sources of error to make sure that the results are accurateWhen a measurement is close to the true value (the actual value) being measured. and repeatableIf an experiment is repeated several times and the results are similar to the measurements obtained previously, the results are repeatable. .

Accuracy can be improved by getting a precise value for the room temperature before the experiment begins, and using an insulated polystyrene cup to stop unwanted heat transfers.

Results are repeatable if they are similar to each other when the experiment is repeated.
Sometimes one result from a set of repeat measurements is very different from the rest of the results. This is called an outlierAn outlier is another name for an anomalous result. A result or measurement is an outlier if it doesn’t show the same pattern as the other results in a set of data collected under the same conditions. or an anomalous resultA result or measurement is anomalous if it doesn’t show the same pattern as the other results in a set of data collected under the same conditions. An anomalous result is also called an outlier. .
Usually it is best to ignore an outlier.
Find out more about accuracy and repeatability in this guide.

Why would it be a good idea to put an insulating lid onto the polystyrene cup when doing this experiment?
It would prevent heat from escaping out of the cup during an exothermic reaction and it would prevent heat from entering the cup in an endothermic reaction.
This makes the results more accurate.

Test your knowledge
Quiz
Working safely in the lab
Find out how to spot risks, hazards and understand hazard symbols

More on Chemical reactions
Find out more by working through a topic
- count5 of 12

- count6 of 12

- count7 of 12
- count8 of 12
