Draft:Osteoarthritis
Osteoarthritis is usually considered a degeneration of joint cartilage and the underlying bone, most common from middle age onward.
Theoretical osteoarthritis
[edit | edit source]
Def. an inflammation "of a joint or joints causing pain and/or disability, swelling and stiffness, and due to various causes such as infection, trauma, degenerative changes or metabolic disorders"[1] is called arthritis.
Def. a "chronic and progressive disease in which the immune system attacks the joints"; "characterised by pain, inflammation and swelling of the joints, stiffness, weakness, loss of mobility and deformity", where tissues "throughout the body can be affected, including the skin, blood vessels, heart, lungs, and muscles"[2] is called rheumatoid arthritis.
Def. a "form of arthritis, affecting mainly older people, caused by chronic degeneration of the cartilage and synovial membrane of the joints, leading to pain and stiffness"[3] is called osteoarthritis.
Chondrocytes
[edit | edit source]Def. a "cell that makes up the tissue of cartilage"[4] is called a chondrocyte.
"[C]hondrocytes, the only cell type present in cartilage, have very low metabolism activity with no ability to repair cartilage. Moreover, unlike all other tissues, articular cartilage, once damaged, cannot respond by a usual inflammatory response because it is non-vascularized and non-innervated."[5]
Once "degraded, cartilage fragments fall into the joint and contact the synovium. Considered foreign bodies, synovial cells react by producing inflammatory mediators, found in synovial fluid. These mediators can activate chondrocytes present in the superficial layer of cartilage, which leads to metalloproteinase synthesis and, eventually, increase cartilage degradation. The mediators can also induce synovial angiogenesis and increase the synthesis of inflammatory cytokines and MMPs by synovial cells themselves (vicious circle). Thus, OA synovitis perpetuates the cartilage degradation."[5]
"The innate immune system, also known as non-specific immune system, comprises the cells and mechanisms that defend the host from infection by other organisms in a non-specific manner. This system is triggered after the binding of pathogen-associated molecular patterns (PAMPs) and danger-associated molecular patterns (DAMPs) on pattern-recognition receptors (PRRs)21,22. Thus, these responses have been studied as predominant features in multiple non-infectious diseases with tissue injury and/or defective repair. PRRs include membrane-associated PRRs (Toll-like receptors [TLRs], the basic signaling receptors of the innate immune system), cytoplasmic PRRs (nucleotide-binding oligomerization domains [NODs], NALPs, RNA helicases) and secreted PRRs (complement receptors, collectins). PAMPs include bacterial and viral ligands and also extracellular matrix molecules. PAMPs are recognized by TLRs and other PRRs. A pioneer study showed that TLRs are increased in level in OA cartilage lesions23. TLR-2 and TLR-4 ligands such as low- molecular-weight hyaluronic acid, fibronectin, tenascin-C and alarmins (S100 proteins, high-mobility group protein B1 [HMGB1]) have been found in OA synovial fluid24e26. These factors can induce catabolic responses in chondrocytes and/or inflammatory responses in synoviocytes. For example, S100A8 and S100A9 proteins are involved in synovial activation and cartilage destruction, and high levels may predict joint destruction in OA27. These results are corroborated by a proteomic analysis revealing that proteins from OA synovial fluid can induce macrophage production of inflammatory cytokines via TLR-4 signaling28. Interestingly, recent data suggest that these events may occur early in the disease, so innate immunity may be a driver of the OA process. Synovial fluid from patients with early OA cartilage damage showed increased fibroblast-like syno- viocyte responses to TLR-2 and TLR-4 ligands28. Increased levels of interleukin-15 (IL-15) protein are found in the synovial fluid of early knee OA patients when compared to end-stage OA, and numbers of CD8 cells within the synovial membrane is correlated with MMP-129."[5]
"Inflammation is triggered by external mediators such as cytokines and proteases, as well as internal cellular mechanisms leading to increased production of inflammatory mediators and lack of elimination of oxidated proteins. These proteins will in turn increase the concentration of reactive oxygen species (ROS) in cells, further adding to the oxidative damage triggering the inflammation58. Interestingly, oxidative stress can promote cell senescence, and in particular chondrocyte senescence59."[5]
"Although OA is a prototypic age-related disease, the specific mechanisms underlying the process remain largely unknown. At the cellular level, senescence can be divided into two main categories: replicative and secretory. Many human cells in culture have a limited proliferative capacity. After a period of vigorous proliferation, the rate of cell division declines (replicative senescence). However, other cell types like chondrocytes have a lower capacity to divide, which leaves little room for replicative senescence. But these cells have high capacity to synthesize soluble mediators. So, secretory senescence should be predominant with aging. This condition has been called the senescence-associated secretory phenotype (SASP) that includes several inflammatory and prodegradative mediators driven by oxidative stress60. Interestingly, the SASP is primarily a delayed response to (epi)genomic damage61. Indeed, IL-1b-stimulated MMP-13 chondrocyte production increases with age, suggesting that aging chondrocytes acquire a SASP62."[5]
Advanced "glycation endproducts (AGEs), produced by a non-enzymatic process in aging tissues, weaken cartilage by modifying its mechanical properties. They can trigger chondrocyte activation by binding to specific receptors present at the surface of the chondrocytes, called RAGE (receptors for AGE). This process can lead to an overproduction of proinflammatory cytokines and MMPs63e65."[5]
"To understand why the incidence of OA increases greatly after menopause, some groups have investigated estrogen regulation. The estrogen receptor is present in chondrocytes, subchondral osteoblasts and synoviocytes66. Its activation by estrogen derivatives has led to controversial results, depending on their concentration. However, the overall effect predominantly leads to inhibition of the expression and secretion of proinflammatory cytokines such as IL-1 into the joint67. Moreover, decreased ovarian function is accompa- nied by a spontaneous increase in level of proinflammatory cytokines in plasma68".[5]
"Any abnormal mechanical stress applied on a joint (stretch, compression, shear stress, hydrostatic pressure) can be converted into activated intracellular signals in joint cells by mechanoreceptors present at the surface of joint cells (ion channels, integrins)69. These signals may eventually lead to the overexpression of inflammatory soluble mediators such as prostaglandins, chemokines and cytokines when a certain threshold is reached70. This is the case for chondrocytes and for subchondral bone cells present in subchondral bone71e74. Intracellularly, the conversion of a mechanical signal to the synthesis of inflammatory mediators is mediated by the activation of inducible signaling pathways. Among them, NF-kB and MAPK pathways seem predominant75."[5]
Synoviocytes
[edit | edit source]Def. a "form of fibroblast cell that occurs in the synovial membrane"[6] is called a synoviocyte.
"The synovial membrane is a thin lining layer within the joint cavity that is responsible for maintaining normal joint function and homeostasis. Within the synovial membrane, the cells most closely associated with this homeostatic function are the normally highly synthetic fibroblast-like synovial (FLS) cells [synoviocytes]. These are the primary source of articular hyaluronic acid and other glycoproteins such as lubricin [1,2]. In chronic inflammatory disorders such as rheumatoid arthritis (RA), the synovial membrane becomes the target of a persistent inflammatory process that leads to fundamental changes in the phenotype and function of FLS cells."[7]
"FLS cells participate in complex autocrine and paracrine activation networks with macrophages, lymphocytes, and dendritic cells, which serve to sustain the synovitis and to enhance its destructive potential."[7]
A "number of proteins that have been implicated in the normal or pathological FLS function (e.g. uridine diphosphoglucose dehydrogenase, galectin 1 and galectin 3) or that have been characterized as potential autoantigens in rheumatoid arthritis (e.g. BiP, colligin, HC gp-39). A novel uncharacterized protein product of chromosome 19 open reading frame 10 was also detected as an apparently major component of FLS cells."[7]
Joints
[edit | edit source]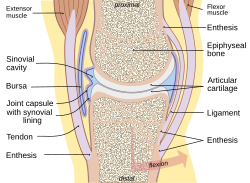
Def. any "part of the body where two bones join, in most cases allowing that part of the body to be bent or straightened"[8] is called a joint.
Prevention
[edit | edit source]Primary prevention for osteoarthritis includes:
- Weight loss
- Physical activity
- Injury prevention
- Control infectious disease
- Avoidance of trauma on the joint
- Omega-3 fatty acid
See also
[edit | edit source]- Arthritis (8 kB) (8 November 2010)
- Deoxyribonucleic acids (36 kB) (1 December 2019)
- Epigenetics (27 kB) (5 November 2019)
- Epigenomes (27 kB) (5 November 2019)
- Genealogy (6 kB) (12 September 2019)
- Genes (23 kB) (4 November 2019)
- Genetics (20 kB) (5 November 2019)
- Gene transcriptions (32 kB) (5 November 2019)
- Genomes (25 kB) (5 November 2019)
- Genomics (13 kB) (5 November 2019)
- Heredity (13 kB) (18 December 2019)
- Medicine (20 kB) (11 February 2020)
- Molecular genetics (11 kB) (5 November 2019)
- Orthomolecular medicine (9 kB) (18 September 2019)
- Osteoarthritis (14 kB) (15 February 2020)
- Phosphate biochemistry (63 kB) (5 November 2019)
- Phosphate budgets (50 kB) (5 November 2019)
- Phosphate reactions (86 kB) (1 September 2019)
- Proteins (26 kB) (1 September 2019)
References
[edit | edit source]- ↑ Jonathan Webley (7 September 2005). "arthritis". San Francisco, California: Wikimedia Foundation, Inc. Retrieved 31 August 2019.
{{cite web}}:|author=has generic name (help) - ↑ Jonathan Webley (7 September 2005). "rheumatoid arthritis". San Francisco, California: Wikimedia Foundation, Inc. Retrieved 31 August 2019.
{{cite web}}:|author=has generic name (help) - ↑ Jonathan Webley (7 September 2005). "osteoarthritis". San Francisco, California: Wikimedia Foundation, Inc. Retrieved 2017-01-14.
{{cite web}}:|author=has generic name (help) - ↑ SemperBlotto (30 November 2008). "chondrocyte". San Francisco, California: Wikimedia Foundation, Inc. Retrieved 2017-01-14.
{{cite web}}:|author=has generic name (help) - ↑ 5.0 5.1 5.2 5.3 5.4 5.5 5.6 5.7 F. Berenbaum (January 2013). "Osteoarthritis as an inflammatory disease (osteoarthritis is not osteoarthrosis!)". Osteoarthritis and Cartilage 21 (1): 16-21. doi:10.1016/j.joca.2012.11.012. PMID 23194896. http://www.oarsijournal.com/article/S1063-4584(12)01025-4/pdf. Retrieved 2017-01-14.
- ↑ SemperBlotto (6 March 2012). synoviocyte. San Francisco, California: Wikimedia Foundation, Inc. https://en.wiktionary.org/wiki/synoviocyte. Retrieved 2017-01-14.
- ↑ 7.0 7.1 7.2 Kumar Dasuri, Mihaela Antonovici, Keding Chen, Ken Wong, Kenneth Standing, Werner Ens, Hani El-Gabalawy, and John A Wilkins (2004). "The synovial proteome: analysis of fibroblast-like synoviocytes". Arthritis Research and Therapy 6 (2): R161–R168. doi:10.1186/ar1153. https://www.ncbi.nlm.nih.gov/pmc/articles/PMC400437/. Retrieved 2017-01-14.
- ↑ Paul G (9 September 2004). "joint". San Francisco, California: Wikimedia Foundation, Inc. Retrieved 2017-01-14.
{{cite web}}:|author=has generic name (help)
External links
[edit | edit source]|
Learn more about Osteoarthritis |
