Draft:Orthomolecular medicine
""Megavitamin therapy" was first coined in 1952 by psychiatrists Humphry Osmond and Abram Hoffer to describe the large dosages of niacin used in the treatment of schizophrenia and mescaline psychosis."[1]
"Megavitamin therapy has become a sub-category of Orthomolecular medicine."[1]
Theoretical orthomolecular medicine
[edit | edit source]Def. "the restoration and maintenance of health through the administration of adequate amounts of substances that are normally present in the body"[2] is called orthomolecular medicine.
Def. a "nutrient or food believed to have curative properties"[3] or a "food used as a drug"[3] is called a nutraceutical.
Alkaloids
[edit | edit source]Historically, Zanthoxylum (Prickly ash) bark was used in traditional medicine.[4]
Species identified in Nigeria contains several types of alkaloids including benzophenanthridines (nitidine, dihydronitidine, oxynitidine, fagaronine, dihydroavicine, chelerythrine, dihydrochelerythrine, methoxychelerythrine, norchelerythrine, oxychelerythrine, decarine and fagaridine), furoquinolines (dictamine, 8-methoxydictamine, skimmianine, 3-dimethylallyl-4-methoxy-2-quinolone), carbazoles (3-methoxycarbazole, glycozoline), aporphines (berberine, tembetarine,[5] magnoflorine, M-methyl-corydine), canthinones (6-canthinone), acridones (1-hydroxy-3-methoxy-10-methylacridon-9-one, 1-hydroxy-10-methylacridon-9-one, zanthozolin), and aromatic and aliphatic amides.[6] Hydroxy-alpha sanshool is a bioactive component of plants from the genus Zanthoxylum, including the Sichuan pepper.
Aporphines
[edit | edit source]Aporphines include berberine and tembetarine found in Prickly ash bark.[5]
Benzophenanthridines
[edit | edit source]Benzophenanthridines incude nitidine, dihydronitidine, oxynitidine, fagaronine, dihydroavicine, chelerythrine, dihydrochelerythrine, methoxychelerythrine, norchelerythrine, oxychelerythrine, decarine and fagaridine found in Prickly ash bark.[5]
Berberines
[edit | edit source]Berberine is a quaternary ammonium salt from the protoberberine group of benzylisoquinoline alkaloids found in such plants as Berberis, such as Berberis vulgaris (barberry), Berberis aristata (tree turmeric), Mahonia aquifolium (Oregon grape), Hydrastis canadensis (goldenseal), Xanthorhiza simplicissima (yellowroot), Phellodendron amurense (Amur cork tree),[7] Coptis chinensis (Chinese goldthread), Tinospora cordifolia, Argemone mexicana (prickly poppy), and Eschscholzia californica (Californian poppy).
Berberine is usually found in the roots, rhizomes, stems, and bark.[8]
The safety of using berberine for any condition is not adequately defined by high-quality clinical research.[9]
Its potential for causing adverse effects is high, including untoward interactions with prescription drugs, reducing the intended effect of established therapies.[9] Berberine inhibits the CYP2D6 and CYP3A4 enzymes which are involved in metabolism of endogenous substances and xenobiotics, including many prescription drugs.[10][11]
It is particularly unsafe for use in children.[9] On the other hand, in May 2021, a comprehensive review article was published that highlighted the efficacy of berberine as a promising anti-oncogenic herpesvirus drug[12]
The dried fruit of Berberis vulgaris is used in herbal medicine.[13] The chemical constituents include isoquinolone alkaloids, especially berberine, with a full list of phytochemicals compiled.[14]
Carbazoles
[edit | edit source]Carbazoles include 3-methoxycarbazole and glycozoline found in Prickly ash bark.[5]
Furoquinolines
[edit | edit source]Furoquinolines include dictamine, 8-methoxydictamine, skimmianine, and 3-dimethylallyl-4-methoxy-2-quinolone found in Prickly ash bark.[5]
Huperzine A
[edit | edit source]Huperzine A is a naturally occurring sesquiterpene alkaloid compound found in the firmoss Huperzia serrata[15] and in varying quantities in other food Huperzia species, including H. elmeri, H. carinat, and H. aqualupian.[16] Huperzine A has been investigated as a treatment for neurological conditions such as Alzheimer's disease, but a meta-analysis of those studies concluded that they were of poor methodological quality and the findings should be interpreted with caution.[17][18]
Huperzine A is extracted from Huperzia serrata.[15] "Huperzine A (HupA), a novel alkaloid isolated from the Chinese herb Huperzia serrata, is a potent, highly specific and reversible inhibitor of acetylcholinesterase (AChE)."[19] It is a reversible acetylcholinesterase inhibitor[19][20][21][22] and NMDA receptor antagonist[23] that crosses the blood-brain barrier.[24] Acetylcholinesterase is an enzyme that catalyzes the breakdown of the neurotransmitter acetylcholine and of some other choline esters that function as neurotransmitters. The structure of the complex of huperzine A with acetylcholinesterase has been determined by X-ray crystallography (PDB code: 1VOT; see the 3D structure).[25]
For some years, huperzine A has been investigated as a possible treatment for diseases characterized by neurodegeneration, particularly Alzheimer's disease.[15][26] A 2013 meta-analysis found that huperzine A may be efficacious in improving cognitive function, global clinical status, and activities of daily living for individuals with Alzheimer's disease. However, due to the poor size and quality of the clinical trials reviewed, huperzine A should not be recommended as a treatment for Alzheimer's disease unless further high quality studies confirm its beneficial effects.[17]
Huperzine A is also marketed as a dietary supplement with claims made for its ability to improve memory and mental function.[27]
Huperzine A has also been noted to help induce lucid dreaming.[28]
Valerian alkaloids
[edit | edit source]Actinidine,[29] chatinine,[29][30] shyanthine,[29] valerianine,[29] and valerine[29]
Alpha-lipoic acids
[edit | edit source]"Alpha-Lipoic Acid is a naturally occurring micronutrient, synthesized in small amounts by plants and animals (including humans), with antioxidant and potential chemopreventive activities. Alpha-lipoic acid acts as a free radical scavenger and assists in repairing oxidative damage and regenerates endogenous antioxidants, including vitamins C and E and glutathione. This agent also promotes glutathione synthesis. In addition, alpha-lipoic acid exerts metal chelating capacities and functions as a cofactor in various mitochondrial enzyme complexes involved in the decarboxylation of alpha-keto acids."[31]
Lipoic acid (LA), also known as α-lipoic acid (ALA) and thioctic acid,[32] is an organosulfur compound derived from caprylic acid (octanoic acid).[33] ALA is made in animals normally, is essential for aerobic metabolism, and is manufactured and available as a dietary supplement in some countries where it is marketed as an antioxidant, and is available as a pharmaceutical drug in other countries.[33]
(R)-(+)-lipoic acid (RLA) may function in vivo like a B-vitamin and at higher doses like plant-derived nutrients, such as curcumin, sulforaphane, resveratrol, and other nutritional substances that induce phase II detoxification enzymes, thus acting as cytoprotective agents.[34][35] This stress response indirectly improves the antioxidant capacity of the cell.[36]
Amino acids
[edit | edit source]GABA
[edit | edit source]GABA is an amino acid, as it has both a primary amine and a carboxylic acid functional group.
GABA is also found in plants. It is the most abundant amino acid in the apoplast of tomatoes.[37] Evidence also suggests a role in cell signalling in plants.[38][39]
L-ornithine
[edit | edit source]In mammalian non-hepatic tissues, the main use of the urea cycle is in arginine biosynthesis, so, as an intermediate in metabolic processes, ornithine is quite important.[40]
L-Ornithine supplementation attenuated fatigue in subjects in a placebo-controlled study using a cycle ergometer. The results suggested that L-ornithine has an antifatigue effect in increasing the efficiency of energy consumption and promoting the excretion of ammonia.[41][42]
L-tyrosine
[edit | edit source]Tyrosine is a precursor to neurotransmitters and increases plasma neurotransmitter levels (particularly dopamine and norepinephrine),[43] but has little if any effect on mood in normal subjects.[44][45][46] However, a number of studies have found tyrosine to be useful during conditions of stress, cold, fatigue (in mice),[47] prolonged work and sleep deprivation,[48][49] with reductions in stress hormone levels,[50] reductions in stress-induced weight loss seen in animal trials,[47] and improvements in cognitive and physical performance[45][51][52] seen in human trials.
Tyrosine does not seem to have any significant effect on cognitive or physical performance in normal circumstances,[53][54] but does help sustain working memory better during multitasking.[55]
Coenzyme Q10
[edit | edit source]"Coenzyme Q10 (ubiquinone, or coQ10) is an antioxidant that is essential for mitochondrial energy production. It is manufactured in the body, but with aging the amounts are inadequate for optimum health. CoQ10 is essential for the heart muscle, and it helps lower blood pressure, improve congestive heart failure, and protect the brain in degenerative conditions such as Parkinson’s and Alzheimer’s diseases (Morisco et al 1993). Statin drugs significantly lower the production of coQ10. Typical supplemental doses of coQ10 range from 100 mg daily for prevention against high blood pressure to 400 mg for heart disease patients (Munkholm et al 1999)."[2]
A meta-analysis of people with heart failure 30–100 mg/d of CoQ10 resulted in 31% lower mortality, exercise capacity was also increased, but no significant difference was found in the endpoints of left heart ejection fraction and New York Heart Association NYHA) classification.[56]
Generally, CoQ10 is well tolerated. The most common side effects are gastrointestinal symptoms (nausea, vomiting, appetite suppression, and abdominal pain), rashes, and headaches.[57]
While there is no established ideal dosage of CoQ10, a typical daily dose is 100–200 milligrams.
The Canadian Headache Society guideline for migraine prophylaxis recommends, based on low-quality evidence, that 300 mg of CoQ10 be offered as a choice for prophylaxis.[58]
Detailed reviews on occurrence of CoQ10 and dietary intake were published in 2010.[59] Besides the endogenous synthesis within organisms, CoQ10 also is supplied to the organism by various foods. Despite the scientific community's great interest in this compound, however, a very limited number of studies have been performed to determine the contents of CoQ10 in dietary components. The first reports on this aspect were published in 1959, but the sensitivity and selectivity of the analytical methods at that time did not allow reliable analyses, especially for products with low concentrations.[59] Since then, developments in analytical chemistry have enabled a more reliable determination of CoQ10 concentrations in various foods:
| Food | CoQ10 concentration (mg/kg) | |
|---|---|---|
| Beef | heart | 113 |
| liver | 39–50 | |
| muscle | 26–40 | |
| Pork | heart | 12–128 |
| liver | 23–54 | |
| muscle | 14–45 | |
| Chicken | breast | 8–17 |
| thigh | 24–25 | |
| wing | 11 | |
| Fish | sardine | 5–64 |
| mackerel: | ||
| – red flesh | 43–67 | |
| – white flesh | 11–16 | |
| salmon | 4–8 | |
| tuna | 5 | |
| vegetable oils | soybean | 54–280 |
| olive | 4–160 | |
| grapeseed | 64–73 | |
| sunflower | 4–15 | |
| canola | 64–73 | |
| Nuts | peanut | 27 |
| walnut | 19 | |
| sesame seed | 18–23 | |
| pistachio | 20 | |
| hazelnut | 17 | |
| almond | 5–14 | |
| Vegetables | parsley | 8–26 |
| broccoli | 6–9 | |
| cauliflower | 2–7 | |
| spinach | up to 10 | |
| Chinese cabbage | 2–5 | |
| Fruit | avocado | 10 |
| blackcurrant | 3 | |
| grape | 6–7 | |
| strawberry | 1 | |
| orange | 1–2 | |
| grapefruit | 1 | |
| apple | 1 | |
| banana | 1 | |
Meat and fish are the richest sources of dietary CoQ10; levels over 50 mg/kg may be found in beef, pork, and Chicken as food|chicken heart and liver. Dairy products are much poorer sources of CoQ10 than animal tissues. Vegetable oils also are quite rich in CoQ10. Within vegetables, parsley and perilla are the richest CoQ10 sources, but significant differences in their CoQ10 levels may be found in the literature. Broccoli, grapes, and cauliflower are modest sources of CoQ10. Most fruit and berries represent a poor to very poor source of CoQ10, with the exception of avocados, which have a relatively high CoQ10 content.[59]
Intake
[edit | edit source]In the developed world, the estimated daily intake of CoQ10 has been determined at 3–6 mg per day, derived primarily from meat.[59]
Cynarines
[edit | edit source]Artichoke contains the bioactive agents apigenin and luteolin.[60]
Def. "any of many compounds that are plant metabolites, being formally derived from flavone; they have antioxidant properties,[61] and sometimes contribute to flavor[62]" is called a flavonoid.
Def. "any of a class of tricyclic aromatic heterocyclic ketones, especially the naturally occurring flavonoids"[63] is called a flavone.
Apigenin (4′,5,7-trihydroxyflavone), found in many plants, is a natural product belonging to the flavone class that is the aglycone of several naturally occurring glycosides.
Apigenin is found in many fruits and vegetables, but parsley, celery, celeriac, and chamomile tea are the most common sources.[64] Apigenin is particularly abundant in the flowers of chamomile plants, constituting 68% of total flavonoids.[65] Dried parsley can contain about 45 mg/gram and dried chamomile flower about 3-5 mg/gram apigenin.[66] The apigenin content of fresh parsley is reportedly 215.5 mg/100 grams, which is much higher than the next highest food source, green celery hearts providing 19.1 mg/100 grams.[67]
Luteolin is a flavone, a type of flavonoid, with a yellow crystalline appearance.[68] Luteolin can function as either an antioxidant or a pro-oxidant and plants rich in luteolin have been used in Chinese traditional medicine[69]
Luteolin is most often found in leaves, but it is also seen in rinds, barks, clover blossom, and ragweed pollen.[68] It has also been isolated from the aromatic flowering plant, Salvia tomentosa in the mint family, Lamiaceae.[70]
Dietary sources include celery, broccoli, artichoke, Bell pepper (green pepper), parsley, thyme, dandelion, perilla, chamomile tea, carrots, olive oil, peppermint, rosemary, navel oranges, and oregano.[71][72] It can also be found in the seeds of the palm Aiphanes aculeata.[73]
The total antioxidant capacity of artichoke flower heads is one of the highest reported for vegetables.[74] Cynarine is a chemical constituent in Cynara. The majority of the cynarine found in artichoke is located in the pulp of the leaves, though dried leaves and stems of artichoke also contain it.
Cynarine is a hydroxycinnamic acid derivative and a biologically active chemical constituent of artichoke (Cynara cardunculus).[75]
Enzymes
[edit | edit source]Enzymes can be classified by two main criteria: either protein primary structure (amino acid sequence) similarity (and thus evolutionary relationship) or enzymatic activity.
Enzyme activity. An enzyme's name is often derived from its substrate or the chemical reaction it catalyzes, with the word ending in -ase.[76] Examples are lactase, alcohol dehydrogenase and DNA polymerase. Different enzymes that catalyze the same chemical reaction are called isozymes.[76]
The International Union of Biochemistry and Molecular Biology have developed a nomenclature for enzymes, the Enzyme Commission number (EC number). Each enzyme is described by "EC" followed by a sequence of four numbers which represent the hierarchy of enzymatic activity (from very general to very specific). That is, the first number broadly classifies the enzyme based on its mechanism while the other digits add more and more specificity.[77]
The top-level classification is:
- EC 1, Oxidoreductases: catalyze oxidation/reduction reactions
- EC 2, Transferases: transfer a functional group (e.g. a methyl or phosphate group)
- EC 3, Hydrolases: catalyze the hydrolysis of various bonds
- EC 4, Lyases: cleave various bonds by means other than hydrolysis and oxidation
- EC 5, Isomerases: catalyze isomerization changes within a single molecule
- EC 6, Ligases: join two molecules with covalent bonds.
These sections are subdivided by other features such as the substrate, products, and chemical mechanism. An enzyme is fully specified by four numerical designations. For example, hexokinase (EC 2.7.1.1) is a transferase (EC 2) that adds a phosphate group (EC 2.7) to a hexose sugar, a molecule containing an alcohol group (EC 2.7.1).[78]
EC categories do not reflect sequence similarity. For instance, two ligases of the same EC number that catalyze exactly the same reaction can have completely different sequences. Independent of their function, enzymes, like any other proteins, have been classified by their sequence similarity into numerous families. These families have been documented in dozens of different protein and protein family databases such as Pfam.[79]
Oxidoreductases
[edit | edit source]15-cis-phytoene desaturases (PDS, plant-type phytoene desaturases) (EC 1.3.5.5, 15-cis-phytoene:plastoquinone oxidoreductase), are enzymes involved in the carotenoid biosynthesis in plants and cyanobacteria.[80] Phytoene desaturases are membrane-bound enzymes localized in plastids and introduce two double bonds into their colorless substrate phytoene by dehydrogenation and isomerize two additional double bonds.[81][82] This reaction starts a biochemical pathway involving three further enzymes (zeta-carotene isomerase, 9,9'-Dicis-zeta-carotene desaturase) and carotene cis-trans isomerase) called the poly-cis pathway and leads to the red colored lycopene. The homologous phytoene desaturase found in bacteria and fungi (Phytoene desaturase (lycopene-forming) CrtI) converts phytoene directly to lycopene by an all-trans pathway.[83]
Fatty acids
[edit | edit source]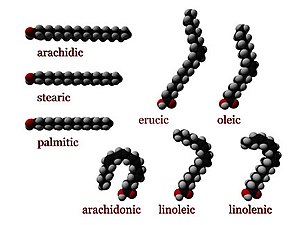
Def. any "of a class of aliphatic carboxylic acids, of general formula CnH2n+1COOH, that occur combined with glycerol as animal or vegetable oils and fats"[84] is called a fatty acid.
"Only those with an even number of carbon atoms are normally found in natural fats"[85]
Usage notes: "The above general formula applies to the saturated fatty acids. Remove 2 hydrogen atoms for an unsaturated fatty acid, and 2 hydrogen atoms for every double bond in a polyunsaturated faty acid."[86]
Flavonoids
[edit | edit source]The raw dandelion flowers contain diverse phytochemicals, including polyphenols, such as flavonoids apigenin, isoquercitrin (a quercetin-like compound), and caffeic acid, as well as terpenoids, triterpenes, and sesquiterpenes.[87] The roots contain a substantial amount of the prebiotic fiber inulin. Dandelion greens contain lutein.[88]
Flavonoids (or bioflavonoids; from the Latin word flavus, meaning yellow, their color in nature) are a class of polyphenolic secondary metabolites found in plants, and thus commonly consumed in diets.[67]
The tartness of cranberry juice derives from its mixed content of polyphenols, including flavonoids, proanthocyanidins, anthocyanins, phenolic acids, and ellagitannins.[89]
Chemically, flavonoids have the general structure of a 15-carbon skeleton, which consists of two phenyl rings (A and B) and a heterocyclic ring (C, the ring containing the embedded oxygen).[67][90] This carbon structure can be abbreviated C6-C3-C6. According to the IUPAC nomenclature,[91][92] they can be classified into:
- flavonoids or bioflavonoids
- isoflavonoids, derived from 3-phenylchromen-4-one (3-phenyl-1,4-benzopyrone) structure
- neoflavonoids, derived from 4-phenylcoumarine (4-phenyl-1,2-benzopyrone) structure
Flavonoids (vitexin, casticin), iridoid glycoside (agnuside, aucubin), p-hydroxybenzoic acid,[93][94] alkaloids, essential oils, fatty oils, diterpenoids and steroids have been identified in the chemical analysis of Vitex agnus-castus.[95] They occur in the fruits and in the leaves.[94]
Flavanones
[edit | edit source]Flavanones: hesperidin,[96] 6-methylapigenin,[96] and linarin[97] occur in Valerian.
Flavonols
[edit | edit source]Def. any "of several flavonoids that have a 3-hydroxyflavone backbone"[98] is called a flavonol.
Herbacetin is a flavonol, a type of flavonoid.
Flavonolignans
[edit | edit source]Rhodiolin, a flavonolignan, is the product of the oxidative coupling of coniferyl alcohol with the 7,8-dihydroxy grouping of herbacetin. It can be found in the rhizome of Rhodiola rosea.[99]
Proanthocyanidins
[edit | edit source]Proanthocyanidins, including the lesser bioactive and bioavailable polymers (four or more catechins) represent a group of condensed flavan-3-ols, such as procyanidins, prodelphinidins and propelargonidins, that can be found in many plants, most notably apples, maritime pine bark and that of most other pine species, cinnamon,[100] aronia fruit, cocoa beans, grape seed, grape skin (procyanidins and prodelphinidins).[101] Cocoa beans contain the highest concentrations.[102]
Proanthocyanidins also may be isolated from Quercus petraea and Quercus robur heartwood (wine barrel oaks).[103] Açaí oil, obtained from the fruit of the açaí palm (Euterpe oleracea), is rich in numerous procyanidin oligomers.[104]
Apples contain on average per serving about eight times the amount of proanthocyanidin found in wine, with some of the highest amounts found in the Red Delicious and Granny Smith varieties.[105]
An extract of maritime pine bark called Pycnogenol bears 65-75 percent proanthocyanidins (procyanidins).[106]
Proanthocyanidin glycosides can be isolated from cocoa liquor.[107]
The seed testas of field beans (Vicia faba) contain proanthocyanidins[108] that affect the digestibility in piglets[109] and could have an inhibitory activity on enzymes.[110] Cistus salviifolius also contains oligomeric proanthocyanidins.[111]
| Dietary source[112] | Proanthocyanidin
(mg/100g) |
|---|---|
| Fruits | |
| Grape seeds | 3532 |
| Blueberries | 332 |
| Apples | 70-141 |
| Pears | 32-42 |
| Nuts | |
| Hazelnuts | 501 |
| Other | |
| Cinnamon bark | 8108 |
| Sorghum grains | 3965 |
| Baking chocolate | 1636 |
| Red wine | 313 |
Grape seeds are rich in unsaturated fatty acids, which helps lowering levels of total cholesterol and LDL cholesterol in the blood.[113]
Quercetin
[edit | edit source]"Quercetin is a flavonoid that helps to control allergy symptoms of rhinitis and sinusitis. It stabilizes the membranes of mast cells, reducing the release of histamine. It is also helpful in lowering the risk of cataract by inhibiting glycoprotein formation in the lens (Cornish, et al 2002). Typical doses of quercetin are 800 mg to 1200 mg daily."[2]
Rutins
[edit | edit source]Rutin (rutoside or rutinoside)[114] and other dietary flavonols are under preliminary clinical research for their potential biological effects, such as in reducing post-thrombotic syndrome, venous insufficiency, or endothelial dysfunction, but there was no high-quality evidence for their safe and effective uses as of 2018.[114][115][116] As a flavonol among similar flavonoids, rutin has low bioavailability due to poor absorption, high metabolism, and rapid excretion that collectively make its potential for use as a therapeutic agent limited.[114]
Glycosides
[edit | edit source]Herbacetin diglucoside can be isolated from flaxseed hulls.[117]
Rhodionin is a herbacetin rhamnoside found in Rhodiola species.[118]
| Glycosides | Aglycone | Glycone | Plants | Genus species |
|---|---|---|---|---|
| Alcohol glycosides | Alcohol | Glycone | Common name | Genus species |
| Rosavin | cinnamyl alcohol | arabinose | Rhodiola | Rhodiola rosea |
| Salicin | salicyl alcohol | glucose | Willow | Salix |
| Salidrosides | tyrosol | glucose | Rhodiola | Rhodiola rosea |
| Anthraquinone glycosides | Anthraquinone derivative | Glycone | Common name | Genus species |
| Sennosides | reduced anthraquinone | glucose | Legume | Senna |
| Chromone glycosides | Benzo-gamma-pyrone | Glycone | Common name | Genus species |
| 3,5,7-trihydroxylchromone-3-O-alpha-L-arabinopyranoside | chromone | arabinose | Rhododendron | Rhododendron spinuliferum |
| Eucryphin | chromone | rhamnoside | Eucryphia | Eucryphia cordifolia |
| Coumarin glycosides | coumarin | Glycone | Common name | Genus species |
| Aesculin | coumarin | glucose | Horse chestnut | Aesculus hippocastanum |
| Cyanogenic glycosides | Cyanogin | Glycone | Common name | Genus species |
| Amygdalin | cyanohydrin | glucose | Apricot kernels | Prunus armeniaca |
| Flavonoid glycosides | Flavonoid | Glycone | Common name | Genus species |
| Hesperidin | Hesperetin | Rutinose | Bitter Orange | Citrus aurantium |
| Naringin | Naringenin | Neohesperidose | Grapefruit | Citrus × paradisi |
| Rutin | Quercetin | Rutinose | Common rue | Ruta graveolens |
| Quercitrin | Quercetin | Rhamnose | American white oak | Quercus alba |
| Iridoid glycosides | iridoid | Glycone | Common name | Genus species |
| Aucubin | cyclopentan-[C]-pyran | glucose | spotted laurel | Aucuba japonica |
| Phenolic glycosides | Phenol | Glycone | Common name | Genus species |
| Arbutin | Phenol | glucose | Common Bearberry | Arctostaphylos ova-ursi |
| Astringin | Piceatannol | glucose | Japanese knotweed | Reynoutria japonica |
| Rhapontigenin | Pinosylvin | glucose | Crimson glory vine | Vitis coignetiae |
| Steroidal glycosides | Steroid | Glycone | Common name | Genus species |
| Digitonin | saraponin | glucose | foxglove | Digitalis purpurea |
| Steviol glycosides | Steviol | Glycone | Common name | Genus species |
| Steviosides | Steviol | isosteviol | candyleaf | Stevia rebaudiana |
| Thioglycosides | Thiod | Glycone | Common name | Genus species |
| Sinigrin | sulfur | glucose | Black mustard | Brassica nigra |
| Triterpene glycosides | Triterpene | Glycone | Common name | Genus species |
| Saponins | Triterpene | glucose | soapbark tree | Quillaja saponaria |
Lagerstroemins
[edit | edit source]Chemical compounds that have been isolated from the extract include corosolic acid, lager-stroemin, flosin B, and reginin A.[119]
Corosolic acid is a pentacyclic triterpene acid found in Lagerstroemia speciosa, similar in structure to ursolic acid, differing only in the fact that it has a 2-alpha-hydroxy attachment.[120]
In Vietnam the plant's young leaves are consumed as vegetables, and its old leaves and mature fruit are used in traditional medicine for reducing glucose in blood.[121]
Banaba plant, Lagerstroemia speciosa (giant crepe-myrtle, Queen's crepe-myrtle, banabá plant, or pride of India[122]) is a species of Lagerstroemia native to tropical southern Asia.
Lignans
[edit | edit source]The lignans are a large group of low molecular weight polyphenols found in plants, particularly seeds, whole grains, and vegetables.[123] The name derives from the Latin word for "wood".[124] Lignans are precursors to phytoestrogens.[123][125] They may play a role as antifeedants in the defense of seeds and plants against herbivores.[126]
- Structures of some lignans
-
Matairesinol, illustrating the debenzylbutyrolactone motif
-
Secoisolariciresinol, illustrating the 9,9'-dihydroxydibenzylbutane motif
-
Justicidin A, illustrating the arylnaphthalene mofif
-
Pinoresinol, illustrating the furanofuran motif
-
Steganacin, illustrating the dibenzocyclooctadienelactone motif
-
Podophyllotoxin, illustrating the aryltetralin motif
Lignans and lignin differ in their molecular weight, the former being small and soluble in water, the latter being high polymers that are undigestable:
- both are polyphenolic substances derived by oxidative coupling of monolignols
- most lignans feature a C18 cores, resulting from the dimerization of C9 precursors
- coupling of the lignols occurs at C8
- classes of lignans: "furofuran, furan, dibenzylbutane, dibenzylbutyrolactone, aryltetralin, arylnaphthalene, dibenzocyclooctadiene, and dibenzylbutyrolactol."[127]
Many lignans are metabolized by mammalian gut microflora, producing enterolignans.[128][129]
Flax seeds and sesame seeds contain high levels of lignans.[123][130]
The principal lignan precursor found in flaxseeds is secoisolariciresinol diglucoside.[123][130]
Other foods containing lignans include cereals (rye, wheat, oat and barley), soybeans, tofu, cruciferous vegetables, such as broccoli and cabbage, and some fruits, particularly apricots and Strawberry|strawberries.[123]
Lignans are not present in seed oil, and their contents in whole or ground seeds may vary according to geographic location, climate, and maturity of the seed crop, and the duration of seed storage.[123]
Secoisolariciresinol and matairesinol were the first plant lignans identified in foods.[123]
Lariciresinol and pinoresinol contribute about 75% to the total lignan intake, whereas secoisolariciresinol and matairesinol contribute only about 25%.[123]
Foods containing lignans:[123][131]
| Source | Lignan amount |
|---|---|
| Flaxseeds | 85.5 mg per oz (28.35 g) |
| Sesame seeds | 11.2 mg per oz |
| Brassica vegetables | cup (125 ml) |
| Strawberries | 0.2 per half cup |
Magnolia
[edit | edit source]The aromatic bark contains magnolol, honokiol, 4-O-methylhonokiol, and obovatol.[132][133][134][135][136][137] Magnolol[138] and honokiol[139] activate the nuclear receptor peroxisome proliferator-activated receptor gamma.
Magnolol is an organic compound, classified as lignan, a bioactive compound found in the bark of the Houpu magnolia (Magnolia officinalis) or in Magnolia grandiflora.[140] The compound exists at the level of a few percent in the bark of species of magnolia, the extracts of which have been used in traditional Chinese and Japanese medicine. In addition to magnolol, related lignans occur in the extracts including honokiol, which is an isomer of magnolol.
It is known to act on the GABAA receptors in rat cells in vitro[141] as well as having antifungal properties.[142] Magnolol has a number of osteoblast-stimulating and osteoclast-inhibiting activities in cell culture and has been suggested as a candidate for screening for anti-osteoporosis activity.[143] It has anti-periodontal disease activity in a rat model.[144] Structural analogues have been studied and found to be strong allosteric modulators of GABAA.[145]
Magnolol is also binding in dimeric mode to PPARγ, acting as an agonist of this nuclear receptor.[146]
Prickly ash bark
[edit | edit source]Historically, Zanthoxylum (Prickly ash) bark was used in traditional medicine.[4]
Plants in the genus Zanthoxylum contain the lignan sesamin.
Minerals
[edit | edit source]Oils
[edit | edit source]Phenylpropanoids
[edit | edit source]

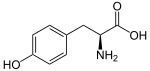
The phenylpropanoids are a diverse family of organic compounds that are synthesized by plants from the amino acids phenylalanine and tyrosine.[147] Their name is derived from the six-carbon, aromatic phenyl group and the three-carbon propene tail of coumaric acid, which is the central intermediate in phenylpropanoid biosynthesis. From 4-coumaroyl-CoA emanates the biosynthesis of myriad natural products including lignols (precursors to lignin and lignocellulose), flavonoids, isoflavonoids, coumarins, aurones, stilbenes, catechin, and phenylpropanoids.[148] The coumaroyl component is produced from cinnamic acid.
Phenylpropanoids are found throughout the plant kingdom, where they serve as essential components of a number of structural polymers, provide protection from ultraviolet light, defend against herbivores and pathogens, and mediate plant-pollinator interactions as floral pigments and scent compounds.
Hydroxycinnamic acids
[edit | edit source]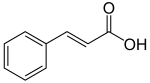
Phenylalanine is first converted to cinnamic acid by the action of the enzyme phenylalanine ammonia-lyase (PAL). Some plants, mainly monocotyledonous, use tyrosine to synthesize p-coumaric acid by the action of the bifunctional enzyme phenylalanine/tyrosine ammonia-lyase (PTAL). A series of enzymatic hydroxylations and methylations leads to coumaric acid, caffeic acid, ferulic acid, 5-hydroxyferulic acid, and sinapic acid. Conversion of these acids to their corresponding esters produces some of the volatile components of herb and flower fragrances, which serve many functions such as attracting pollinators. Ethyl cinnamate is a common example.
Cinnamic aldehydes and monolignols
[edit | edit source]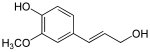

Reduction of the carboxylic acid functional groups in the cinnamic acids provides the corresponding aldehydes, such as cinnamaldehyde. Further reduction provides monolignols including coumaryl alcohol, coniferyl alcohol, and sinapyl alcohol, which vary only in their degree of methoxylation. The monolignols are monomers that are polymerized to generate various forms of lignin and suberin, which are used as a structural component of plant cell walls.
The phenylpropenes, including eugenol, chavicol, safrole and estragole, are also derived from the monolignols. These compounds are the primary constituents of various essential oils.
Coumarins and flavonoids
[edit | edit source]
Hydroxylation of cinnamic acid in the 4-position by trans-cinnamate 4-monooxygenase leads to p-coumaric acid]], which can be further modified into hydroxylated derivatives such as umbelliferone. Another use of p-coumaric acid via its thioester with coenzyme A, i.e. 4-coumaroyl-CoA, is the production of chalcones. This is achieved with the addition of 3 malonyl-CoA molecules and their cyclization into a second phenyl group. Chalcones are the precursors of all flavonoids, a diverse class of phytochemicals.
Stilbenoids
[edit | edit source]
Stilbenoids, such as resveratrol, are hydroxylated derivatives of stilbene. They are formed through an alternative cyclization of cinnamoyl-CoA or 4-coumaroyl-CoA.
A 2018 meta-analysis found no effect of resveratrol on systolic or diastolic blood pressure; a sub-analysis revealed a 2 mmHg decrease in systolic pressure only from resveratrol doses of 300 mg per day, and only in diabetic people.[149] A 2014 Chinese meta-analysis found no effect on systolic or diastolic blood pressure; a sub-analysis found an 11.90 mmHg reduction in systolic blood pressure from resveratrol doses of 150 mg per day.[150]
One review found limited evidence that resveratrol lowered fasting plasma glucose in people with diabetes.[151] Two reviews indicated that resveratrol supplementation may reduce body weight and body mass index, but not fat mass or total blood cholesterol.[152][153] A 2018 review found that resveratrol supplementation may reduce biomarkers of inflammation, TNF-α and C-reactive protein.[154]
Sporopollenin
[edit | edit source]Phenylpropanoids and other natural phenols are part of the chemical composition of sporopollenin. It is related to cutin and suberin.[148] This ill-defined substance found in pollen is unusually resistant to degradation. Analyses have revealed a mixture of biopolymers, containing mainly hydroxylated fatty acids, phenylpropanoids, phenolics and traces of carotenoids. Tracer experiments have shown that phenylalanine is a major precursor, but other carbon sources also contribute. It is likely that sporopollenin is derived from several precursors that are chemically cross-linked to form a rigid structure.
Polycyclic polyprenylated acylphloroglucinols
[edit | edit source]Hyperforin, a phytochemical constituent of the species Hypericum perforatum, known as perforate St John's-wort,[155], is a unique PPAP because it consists of a C8 quaternary stereocenter which was a synthetic challenge unlike other PPAP synthetic targets.[156][157][158] The structure of hyperforin was elucidated by a research group from the Shemyakin Institute of Bio-organic Chemistry (USSR Academy of Sciences in Moscow) and published in 1975.[159][160] A total synthesis of the non-natural hyperforin enantiomer was reported in 2010 which required approximately 50 synthetic transformations and relied heavily on basic organic reactivity.[161] In 2010, an enantioselective total synthesis of the correct enantiomer was disclosed. The retrosynthetic analysis was inspired by hyperforin's structural symmetry and biosynthetic pathway. The synthetic route undertaken generated a prostereogenic intermediate which then established the synthetically challenging C8 stereocenter and facilitated the stereochemical outcomes for the remainder of the synthesis.[158]
Hyperforin is unstable in the presence of light and oxygen.[162] Frequent oxidized forms contain a C3 to C9 hemiketal/heterocyclic bridge or will form furan/pyran derivatives.[163][164]
Polypeptide-p
[edit | edit source]"Polypeptide-p is a very effective hypoglycemic agent when administered subcutaneously to gerbils, langurs, and humans."[165]
An "active principle [polypeptide-p was isolated] from fruits, seeds, and tissue culture of [bitter gourd or melon Momordica charantia] (6, 7)."[165]
Polyphenols
[edit | edit source]Saponins
[edit | edit source]Saponins occur naturally in plants as glycosides and have foam forming properties.[166]
"The seeds of Trigonella foenum graecum (fenugreek) have been reported to have antidiabetic and hypocholesterolaemic properties in both animal models and humans. Activity has been attributed largely to fenugreek's saponin and high fibre content, and is probably not related to its major alkaloid trigonelline. Antihyperglycaemic effects have been linked to delayed gastric emptying caused by the fibre content, and to (unidentified) components that inhibit carbohydrate digestive enzymes. Fenugreek administration may increase plasma insulin levels in vivo. Its major free amino acid, 4-hydroxyisoleucine, stimulates insulin secretion from perfused pancreas in vitro. The hypocholesterolaemic effect has been attributed to increased conversion of hepatic cholesterol to bile salts due to loss, in the faeces, of complexes of these substances with fenugreek fibre and saponins. Fenugreek treatment selectively reduces the LDL and VLDL fractions of total cholesterol, and HDL-cholesterol has also been reported to increase in alloxan-induced diabetic rats and type II diabetic individuals following treatment with fenugreek. Fenugreek administration has not been reported to cause any toxicological effects. Its regular consumption may therefore be beneficial in the management of diabetes and the prevention of atherosclerosis and coronary heart disease."[167]
Diosgenin, a steroidal sapogenin, is reported from Smilax menispermoidea.[168] "Other active compounds reported from various greenbrier species are Parillin (also sarsaparillin or smilacin), sarsapic acid, sarsapogenin and sarsaponin".[169]
Aescins
[edit | edit source]The main active compound of aescin (Horse chestnut seed extract)) is β-aescin, although the mixture also contains various other components including α-aescin, protoescigenin, barringtogenol, cryptoescin and benzopyrones.[170]
Aescin, especially pure β-aescin, may be a safe and effective treatment for short-term treatment of chronic venous insufficiency;[171][172] however, more high quality randomized controlled trials are required to confirm the effectiveness.[172] Horse chestnut extract may be as effective and well tolerated as the use of compression stockings.[172]
Aescin induces endothelial nitric oxide synthesis by making endothelial cells more permeable to calcium ions, and also induces release of prostaglandin F2α.[173][174][175] Other possible mechanisms include serotonin antagonism and histamine antagonism and reduced catabolism of tissue mucopolysaccharides.[173]
Bacopasides
[edit | edit source]Bacopasides are triterpene saponins isolated from Bacopa monnieri.
Members of this class of compounds include:
- Bacopaside I, shows antidepressant-like effects in a mouse model[176]
- Bacopaside II[177]
- Bacopaside III[178][179]
- Bacopaside IV[179]
- Bacopaside V[179]
- Bacopaside VI[180]
- Bacopaside VII[180]
- Bacopaside VIII[180]
- Bacopaside IX[181]
- Bacopaside X[181]
- Bacopaside XI, shows nootropic activity in a mouse model[181]
Ruscosaponins
[edit | edit source]The major phytochemicals in butcher's broom are steroidal saponins.[182] The specific saponins found in butcher's broom are ruscogenins, ruscogenen and neoruscogenin, named for the genus Ruscus.[183] Ruscogenins function as anti-inflammatory agents[184] and are also believed to cause constriction in veins.[185] Currently the mode of action of ruscogenins is not well understood, but one proposed mechanism suggests that ruscogenins suppresses leukocyte migration through both protein and mRNA regulation.[184] Neoruscogenin has been identified as a potent and high-affinity agonist of the nuclear receptor RORα (NR1F1).[186]
Newer research has also uncovered that there are polyphenols present in butcher's broom may also be physiologically active, possibly as an antioxidant.[187][188]
Silybum marianum
[edit | edit source]Def. a "mixture of flavonolignans extracted from the blessed milk thistle (Silybum marianum), used as a source of silibinin"[189] is called a silymarin.
Silibinin (International Nonproprietary Name (INN)), also known as silybin (both from Silybum, the generic name of the plant from which it is extracted), is the major active constituent of silymarin, a standardized extract of the Silybum marianum (milk thistle) seeds, containing a mixture of flavonolignans consisting of silibinin, isosilibinin, silychristin, silidianin, and others. Silibinin itself is a mixture of two diastereomers, silybin A and silybin B, in approximately equimolar ratio.[190] The mixture exhibits a number of pharmacological effects, particularly in the fatty liver, non-alcoholic fatty liver, non-alcoholic steatohepatitis, and there is great clinical evidence for the use of silibinin as a supportive element in alcoholic and Child–Pugh grade 'A', liver cirrhosis.[191] However, despite its several beneficial effects on the liver, silibinin and all the other compounds found in silymarin, especially silychristin seem to act as potent disruptors of the thyroid system by blocking the Monocarboxylate transporter 8 (MCT8) transporter.[192] The long term intake of silymarin can lead to some form of thyroid disease and if taken during pregnancy, silymarin can cause the development of the Allan–Herndon–Dudley syndrome.[192] Although this information is unfortunately still not being taken into consideration by all regulatory bodies, several studies now consider silymarin and especially silychristin to be important inhibitors of the MCT8 transporter and a potential disruptor of the thyroid hormone functions.[192]
Silipide (trade name Siliphos, not to be confused with the water treatment compound of the same name, a glass-like polyphosphate containing sodium, calcium magnesium and silicate, formulated for the treatment of water problems), a complex of silymarin and phosphatidylcholine (lecithin), is about 10 times more bioavailable than silymarin.[193] An earlier study had concluded Siliphos to have 4.6 fold higher bioavailability.[194] It has been also reported that silymarin inclusion complex with β-cyclodextrin is much more soluble than silymarin itself.[195] There have also been prepared glycosides of silybin, which show better water solubility and even stronger hepatoprotective effect.[196]
Silymarin, like other flavonoid]]s, has been shown to inhibit P-glycoprotein-mediated cellular efflux.[197] It has been reported that silymarin inhibits cytochrome P450 enzymes and an interaction with drugs primarily cleared by P450s cannot be excluded.[198]
All of the flavonolignan compounds found in the silymarin mixture seem to block the uptake of thyroid hormones into the cells by selectively blocking the Monocarboxylate transporter 8 (MCT8) transmembrane transporter.[192] The authors of this study noted that especially silychristin, one of the compounds of the silymarin mixture seems to be perhaps the most powerful and selective inhibitor for the (MCT8) transporter.[192] Due to the essential role played by the thyroid hormone in human metabolism in general it is believed that the intake of silymarin can lead to thyroid disease (disruptions of the thyroid system).[192] Because the thyroid hormones and the MCT8 as well are known to play a critical role during early and fetal development, the administration of silymarin during pregnancy is especially thought to be dangerous, potentially leading to the Allan–Herndon–Dudley syndrome, a brain development disorder that causes both moderate to severe intellectual disability and problems with speech and movement.[199]
A phase I clinical trial in humans with prostate cancer designed to study the effects of high dose silibinin found 13 grams daily to be well tolerated in patients with advanced prostate cancer with asymptomatic liver toxicity (hyperbilirubinemia and elevation of alanine aminotransferase) being the most commonly seen adverse event.[200]
Silymarin is also devoid of embryotoxic potential in animal models.[201] [202]
For approved drug preparations and parenteral applications in the treatment of Amanita mushroom poisoning, the water-soluble silibinin-C-2',3-dihyrogensuccinate disodium salt is used and in 2011, it also received Orphan Medicinal Product Designation for the prevention of recurrent hepatitis C in liver transplant recipients by the European Commission.[203]
Silibinin is under investigation to see whether it may have a role in cancer treatment (e.g. due to its inhibition of STAT3 signalling).[204]
Silibinin has a number of potential mechanisms that could benefit the skin: chemoprotective effects from environmental toxins, anti-inflammatory effects, protection from UV induced photocarcinogenesis, protection from sunburn, protection from Ultraviolet (UVB)-induced epidermal hyperplasia, and DNA repair for UV induced DNA damage (double strand breaks).[205] Studies on mice demonstrate a significant protection on chronic unpredictable mild stress (CUMS) induced depressive-like behavior on mice[206] and increased cognition in aged rats as a result of consuming silymarin.[207]
Due to its immunomodulatory,[208] iron chelating and antioxidant properties, this herb has the potential to be used in beta-thalassemia patients who receive regular blood transfusions and suffer from iron overload.[209]
Silymarin can be produced in callus and cells suspensions of Silybum marianum and substituted pyrazinecarboxamides can be used as abiotic elicitors of flavolignan production.[210]
The biosynthesis of silibinin A and silibinin B is composed of two major parts, taxifolin and coniferyl alcohol.[211][212] Coniferyl alcohol is synthesized in milk thistle seed coat, starting with the transformation of phenylalanine into cinnamic acid mediated by phenylalanine ammonia-lyase (PAL).[213]
St. John's wort
[edit | edit source]
The plant contains the following:[214][215]
- Flavonoids (e.g. epigallocatechin, rutin, hyperoside, isoquercetin, quercitrin, quercetin, amentoflavone, biapigenin, astilbin, myricetin, miquelianin, kaempferol, luteolin)
- Phenolic acids (e.g. chlorogenic acid, caffeic acid, p-coumaric acid, ferulic acid, p-hydroxybenzoic acid, vanillic acid)
- Naphthodianthrones (e.g. hypericin, pseudohypericin, protohypericin, protopseudohypericin)
- Phloroglucinols (e.g. hyperforin, adhyperforin)
- Tannins (unspecified, proanthocyanidins reported)
- Volatile oils (e.g. 2-methyloctane, nonane, 2-methyldecane, undecane, α-pinene, β-pinene, α-terpineol, geraniol, myrcene, limonene, caryophyllene, humulene)
- Saturated fatty acids (e.g. isovaleric acid (3-methylbutanoic acid), myristic acid, palmitic acid, stearic acid)
- Alkanols (e.g. 1-tetracosanol, 1-hexacosanol)
- Vitamins & their analogues (e.g. carotenoids, choline, nicotinamide, nicotinic acid)
- Miscellaneous others (e.g. pectin, β-sitosterol, hexadecane, triacontane, kielcorin, norathyriol)
The naphthodianthrones hypericin and pseudohypericin along with the phloroglucinol derivative hyperforin are thought to be among the numerous active constituents.[216][217][218][219] It also contains essential oils composed mainly of sesquiterpenes.[216]
-
Hypericin
-
Pseudohypericin
-
Adhyperforin
-
Hyperforin
-
Amentoflavone
-
Hyperoside
-
Rutin
-
Kaempferol
-
Myricetin
-
Quercetin
-
Quercitrin
-
Isoquercitrin
-
Luteolin
-
Catechin
-
Epicatechin
-
Epigallocatechin
-
Chlorogenic acid
-
Caffeic acid
-
Kielcorin
-
Norathyriol
Temperatures
[edit | edit source]Both energy metabolism [220][221] and body temperature [222][223] increases are observed in humans following extracted capsinoids [Cayenne pepper] or CH-19 Sweet administration. Animal studies also demonstrate these increases, as well as suppressed in body fat accumulation following capsinoids intake.[224][225] The exact mechanisms and the relative importance of each remain under investigation, as are the effects of capsinoids on appetite and satiation.[226]
Terpenoids
[edit | edit source]Vitamins
[edit | edit source]Waxes
[edit | edit source]History
[edit | edit source]"The term "Orthomolecular" was first utilized by two-time Nobel Laureate Linus Pauling in 1968 to characterize the treatment of disease with nutrients that were endogenous to the human body. Orthomolecular simply means "correct molecule" which translates into "essential nutrient". Orthomolecular physicians treat disease by varying the dosages of "correct molecules" which are required but not synthesized by the human body. Doctors, adjusting diet, by eliminating junk foods, and prescribing mega dosages of essential vitamins, minerals, trace metals, amino acids, and fats can correct the chemical imbalances of disease."[1]
See also
[edit | edit source]- Amino acids (32 kB) (6 November 2019)
- Biology (25 kB) (15 January 2020)
- Chemicals (190 kB) (18 February 2020)
- Control groups (43 kB) (24 November 2019)
- Cytokinesis (14 kB) (29 October 2019)
- Deoxyribonucleic acids (48 kB) (29 February 2020)
- Epigenetics (41 kB) (18 January 2020)
- Epigenomes (30 kB) (5 November 2019)
- Eukaryotes (12 kB) (5 November 2019)
- Evolution (41 kB) (18 January 2020)
- Genealogy (10 kB) (29 February 2020)
- Genes (42 kB) (14 March 2020)
- Genetics (23 kB) (5 November 2019)
- Gene transcriptions (36 kB) (5 November 2019)
- Genomes (29 kB) (5 November 2019)
- Genomics (16 kB) (5 November 2019)
- Glandular system (3 kB) (23 May 2014)
- Hair colors (12 kB) (30 November 2018)
- Heredity (13 kB) (24 December 2019)
- How-to's (3 kB) (2 July 2019)
- Human RNA (35 kB) (5 November 2019)
- Human skin pigmentation (4 kB) (16 August 2010)
- Human teeth (31 kB) (19 January 2020)
- Lamarckism (24 kB) (19 January 2020)
- Liquids (39 kB) (11 February 2020)
- Mammalogy (31 kB) (4 March 2020)
- Medicine (20 kB) (11 February 2020)
- Melanocytes (54 kB) (1 February 2020)
- Minerals
- Molecular genetics (14 kB) (5 November 2019)
- Neutraceuticals
- Pigmented lesions of the oromucosa (16 kB) (28 June 2016)
- Orthomolecular medicine (10 kB) (16 March 2020)
- Osteoarthritis (14 kB) (15 February 2020)
- Phosphate biochemistry (63 kB) (5 November 2019)
- Phosphate budgets (50 kB) (5 November 2019)
- Phosphate reactions (87 kB) (17 February 2020)
- Proof of concepts (24 kB) (19 January 2020)
- Proteins (28 kB) (18 February 2020)
- Safety (15 kB) (31 December 2019)
- Spaceflights (24 kB) (31 December 2019)
- Stroke management (12 kB) (4 November 2019)
- Teeth (25 kB) (9 October 2019)
- Terpenoids
- Two-word terms (29 kB) (7 March 2020)
- Waxes
- What is a human? (26 kB) (24 February 2020)
References
[edit | edit source]- ↑ 1.0 1.1 1.2 Eric Braverman (1979). "Orthomolecular Medicine and Megavitamin Therapy: Future and Philosophy". Orthomolecular Psychiatry 8 (4): 265-72. http://www.orthomolecular.org/library/jom/1979/pdf/1979-v08n04-p265.pdf. Retrieved 2014-08-20.
- ↑ 2.0 2.1 2.2 Michael Janson (September 2006). "Orthomolecular medicine: the therapeutic use of dietary supplements for anti-aging". Clinical Interventions in Aging 1 (3): 261-5. PMID 18046879. https://www.ncbi.nlm.nih.gov/pmc/articles/PMC2695174/. Retrieved 25 September 2018.
- ↑ 3.0 3.1 Dvortygirl (23 March 2005). "nutraceutical". San Francisco, California: Wikimedia Foundation, Inc. Retrieved 26 June 2021.
{{cite web}}:|author=has generic name (help) - ↑ 4.0 4.1 Wilbur, C. Keith, MD. Revolutionary Medicine 1700-1800. The Globe Pequot Press. Page 23. 1980.
- ↑ 5.0 5.1 5.2 5.3 5.4 "{title}". Retrieved 2017-05-18.
- ↑ The Nigerian Zanthoxylum; Chemical and biological values. S. K. Adesina, Afr. J. Trad. CAM, 2005, volume 2, issue 3, pages 282-301 ([1] |date=2016-03-03 }})
- ↑ "Simultaneous determination of jatrorrhizine, palmatine, berberine, and obacunone in Phellodendri Amurensis Cortex by RP-HPLC". Zhongguo Zhong Yao Za Zhi = Zhongguo Zhongyao Zazhi = China Journal of Chinese Materia Medica 35 (16): 2061–4. 2010. doi:10.4268/cjcmm20101603. PMID 21046728.
- ↑ "Berberine". PubChem, National Library of Medicine, US National Institutes of Health. March 9, 2020. Retrieved March 10, 2020.
- ↑ 9.0 9.1 9.2 "Berberine: MedlinePlus Supplements". MedlinePlus, National Library of Medicine, US National Institutes of Health. 19 January 2019. Retrieved 15 February 2019.
- ↑ "Clinical evidence of herbal drugs as perpetrators of pharmacokinetic drug interactions". Planta Medica 78 (13): 1458–77. September 2012. doi:10.1055/s-0032-1315117. PMID 22855269.
- ↑ "The enhancement of cardiotoxicity that results from inhibiton of CYP 3A4 activity and hERG channel by berberine in combination with statins". Chemico-biological Interactions 293: 115–123. September 2018. doi:10.1016/j.cbi.2018.07.022. PMID 30086269.
- ↑ Šudomová, M.; Berchová-Bímová, K.; Marzocco, S.; Liskova, A.; Kubatka, P.; Hassan, S.T.S. Berberine in Human Oncogenic Herpesvirus Infections and Their Linked Cancers. Viruses 2021, 13, 1014. https://doi.org/10.3390/v13061014
- ↑ See e.g. "Barberry" @ Alternative Medicine @ University of Maryland Medical Center
- ↑ Mokhber-Dezfuli N, Saeidnia S, Gohari AR, Kurepaz-Mahmoodabadi M. Phytochemistry and pharmacology of berberis species. Pharmacogn Rev. 2014;8(15):8–15. doi:10.4103/0973-7847.125517
- ↑ 15.0 15.1 15.2 Zangara, A (2003). "The psychopharmacology of huperzine A: an alkaloid with cognitive enhancing and neuroprotective properties of interest in the treatment of Alzheimer's disease". Pharmacology Biochemistry and Behavior 75 (3): 675–686. doi:10.1016/S0091-3057(03)00111-4. PMID 12895686.
- ↑ Lim, WH; Goodger, JQ; Field, AR; Holtum, JA; Woodrow, IE (2010). "Huperzine alkaloids from Australasian and southeast Asian Huperzia". Pharmaceutical Biology 48 (9): 1073–1078. doi:10.3109/13880209.2010.485619. PMID 20731560.
- ↑ 17.0 17.1 Yang, Guoyan; Wang, Yuyi; Tian, Jinzhou; Liu, Jian-Ping (2013). "Huperzine A for Alzheimer's Disease: A Systematic Review and Meta-Analysis of Randomized Clinical Trials". PLOS ONE 8 (9): e74916. doi:10.1371/journal.pone.0074916. PMID 24086396. PMC 3781107. //www.ncbi.nlm.nih.gov/pmc/articles/PMC3781107/.
- ↑ Li, J; Wu, HM; Zhou, RL; Liu, GJ; Dong, BR (16 April 2008). "Huperzine A for Alzheimer's disease". Cochrane Database of Systematic Reviews CD005592 (2): CD005592. doi:10.1002/14651858.CD005592.pub2. PMID 18425924.
- ↑ 19.0 19.1 Wang, R; Yan, H; Tang, XC (January 2006). "Progress in studies of huperzine A, a natural cholinesterase inhibitor from Chinese herbal medicine". Acta Pharmacologica Sinica 27 (1): 1–26. doi:10.1111/j.1745-7254.2006.00255.x. PMID 16364207. http://www.chinaphar.com/article/view/3574. Retrieved 6 December 2017.
- ↑ Meletis, Chris D.; Jason E. Barke (2004). Herbs and Nutrients for the Mind: A Guide to Natural Brain Enhancers. Greenwood Publishing Group. pp. 191. ISBN 978-0275983949. https://books.google.com/books?id=a5AMBY9ekzcC&q=Huperzine&pg=PA191.
- ↑ Wang, BS; Wang, H; Wei, ZH; Song, YY; Zhang, L; Chen, HZ (2009). "Efficacy and safety of natural acetylcholinesterase inhibitor huperzine A in the treatment of Alzheimer's disease: an updated meta-analysis". Journal of Neural Transmission 116 (4): 457–465. doi:10.1007/s00702-009-0189-x. PMID 19221692. https://www.scribd.com/doc/295031747/Efficacy-and-Safety-of-Natural-Acetylcholinesterase-Inhibitor-Huperzine-a-in-the-Treatment-of-Alzheimer-s-Disease-An-Updated-Meta-Analysis.
- ↑ Tang, X.C.; He, X.C.; Bai, D.L. (1999). "Huperzine A: A novel acetylcholinesterase inhibitor". Drugs of the Future 24 (6): 647. doi:10.1358/dof.1999.024.06.545143.
- ↑ Coleman, BR; Ratcliffe, RH; Oguntayo, SA; Shi, X; Doctor, BP; Gordon, RK; Nambiar, MP (2008). "[+]-Huperzine A treatment protects against N-methyl-d-aspartate-induced seizure/status epilepticus in rats". Chemico-Biological Interactions 175 (1–3): 387–395. doi:10.1016/j.cbi.2008.05.023. PMID 18588864.
- ↑ Patocka, J (1998). "Huperzine A - an interesting anticholinesterase compound from the Chinese herbal medicine". Acta Medica 41 (4): 155–7. doi:10.14712/18059694.2019.181. PMID 9951045. ftp://orbis.lfhk.cuni.cz/Acta_Medica/1998/!AM498.PDF.
- ↑ Raves, ML; Harel, M; Pang, YP; Silman, I; Kozikowski, AP; Sussman, JL (1997). "Structure of acetylcholinesterase complexed with the nootropic alkaloid, (–)-huperzine A". Nature Structural & Molecular Biology 4 (1): 57–63. doi:10.1038/nsb0197-57. PMID 8989325.
- ↑ Bai, DL; Tang, XC; He, XC (2000). "Huperzine A, A Potential Therapeutic Agent for Treatment of Alzheimer's Disease". Current Medicinal Chemistry 7 (3): 355–374. doi:10.2174/0929867003375281. PMID 10637369.
- ↑ Talbott, SM (2012). Huperzine A (HupA). Routledge. pp. 304–. ISBN 978-1-136-80570-7. https://books.google.com/books?id=9ZZrW_j9XrcC&pg=PA304.
- ↑ "Lucid Dreaming: A Beginner's Guide". The Four Hour Work Week. Retrieved 29 December 2016.
- ↑ 29.0 29.1 29.2 29.3 29.4 Fereidoon Shahidi and Marian Naczk (2013-06-24). Phenolics in food and nutraceuticals. Boca Raton, Florida, USA: CRC Press. ISBN 1-58716-138-9. https://web.archive.org/web/20130624105109/http://books.google.com/books?id=vHOJKw4umikC&pg=PA313.
- ↑ S. Waliszewski (April 10, 1891). "Chatinine, alcaloïde de la racine de valériane". American Journal of Pharmacy 66. https://web.archive.org/web/20130619055528/http://books.google.com/books?id=aPkKAAAAYAAJ&pg=PA166.
- ↑ National Center for Biotechnology Information. "Lipoic Acid". NCBI. Retrieved October 18, 2018.
- ↑ Reljanovic, M; Reichel, G; Rett, K; Lobisch, M et al. (September 1999). "Treatment of diabetic polyneuropathy with the antioxidant thioctic acid (alpha-lipoic acid): A two year multicenter randomized double-blind placebo-controlled trial (ALADIN II). Alpha Lipoic Acid in Diabetic Neuropathy". Free Radical Research 31 (3): 171–9. doi:10.1080/10715769900300721. PMID 10499773.
- ↑ 33.0 33.1 "Lipoic acid". Micronutrient Information Center, Linus Pauling Institute, Oregon State University, Corvallis. 1 January 2019. Retrieved 5 November 2019.
- ↑ Shay, KP; Shenvi, S; Hagen, TM. "Ch. 14 Lipoic Acid as an Inducer of Phase II Detoxification Enzymes Through Activation of Nr-f2 Dependent Gene Expression". Lipoic Acid: Energy Production, Antioxidant Activity and Health Effects. pp. 349–71. In Packer & Patel 2008.
- ↑ Lii, CK; Liu, KL; Cheng, YP; Lin, AH; Chen, HW; Tsai, CW (May 2010). "Sulforaphane and alpha-lipoic acid upregulate the expression of the pi class of glutathione S-transferase through c-jun and Nrf2 activation". Journal of Nutrition 140 (5): 885–92. doi:10.3945/jn.110.121418. PMID 20237067.
- ↑ Shay, KP; Moreau, RF; Smith, EJ; Hagen, TM (June 2008). "Is alpha-lipoic acid a scavenger of reactive oxygen species in vivo? Evidence for its initiation of stress signaling pathways that promote endogenous antioxidant capacity". IUBMB Life 60 (6): 362–7. doi:10.1002/iub.40. PMID 18409172.
- ↑ "Mutations in γ-aminobutyric acid (GABA) transaminase genes in plants or Pseudomonas syringae reduce bacterial virulence". Plant J. 64 (2): 318–30. October 2010. doi:10.1111/j.1365-313X.2010.04327.x. PMID 21070411.
- ↑ "GABA in plants: just a metabolite?". Trends Plant Sci. 9 (3): 110–5. March 2004. doi:10.1016/j.tplants.2004.01.006. PMID 15003233.
- ↑ "Does GABA Act as a Signal in Plants?: Hints from Molecular Studies". Plant Signal Behav 2 (5): 408–9. September 2007. doi:10.4161/psb.2.5.4335. PMID 19704616. PMC 2634229. //www.ncbi.nlm.nih.gov/pmc/articles/PMC2634229/.
- ↑ Weber AL, Miller SL (1981). "Reasons for the occurrence of the twenty coded protein amino acids". Journal of Molecular Evolution 17 (5): 273–84. doi:10.1007/BF01795749. PMID 7277510. http://physwww.mcmaster.ca/~higgsp/3D03/WeberReasons.pdf.
- ↑ Sugino T, Shirai T, Kajimoto Y, Kajimoto O (November 2008). "L-ornithine supplementation attenuates physical fatigue in healthy volunteers by modulating lipid and amino acid metabolism". Nutrition Research 28 (11): 738–43. doi:10.1016/j.nutres.2008.08.008. PMID 19083482.
- ↑ Demura S, Yamada T, Yamaji S, Komatsu M, Morishita K (October 2010). "The effect of L-ornithine hydrochloride ingestion on performance during incremental exhaustive ergometer bicycle exercise and ammonia metabolism during and after exercise". European Journal of Clinical Nutrition 64 (10): 1166–71. doi:10.1038/ejcn.2010.149. PMID 20717126.
- ↑ "Effects of tyrosine and tryptophan ingestion on plasma catecholamine and 3,4-dihydroxyphenylacetic acid concentrations". The Journal of Clinical Endocrinology and Metabolism 57 (4): 760–3. October 1983. doi:10.1210/jcem-57-4-760. PMID 6885965.
- ↑ Leathwood PD, Pollet P (1982). "Diet-induced mood changes in normal populations". Journal of Psychiatric Research 17 (2): 147–54. doi:10.1016/0022-3956(82)90016-4. PMID 6764931.
- ↑ 45.0 45.1 Deijen JB, Orlebeke JF (1994). "Effect of tyrosine on cognitive function and blood pressure under stress". Brain Research Bulletin 33 (3): 319–23. doi:10.1016/0361-9230(94)90200-3. PMID 8293316.
- ↑ Lieberman HR, Corkin S, Spring BJ, Wurtman RJ, Growdon JH (August 1985). "The effects of dietary neurotransmitter precursors on human behavior". The American Journal of Clinical Nutrition 42 (2): 366–70. doi:10.1093/ajcn/42.2.366. PMID 4025206.
- ↑ 47.0 47.1 Hao S, Avraham Y, Bonne O, Berry EM (February 2001). "Separation-induced body weight loss, impairment in alternation behavior, and autonomic tone: effects of tyrosine". Pharmacology, Biochemistry, and Behavior 68 (2): 273–81. doi:10.1016/S0091-3057(00)00448-2. PMID 11267632.
- ↑ Magill RA, Waters WF, Bray GA, Júlia Volaufová, Smith SR, Lieberman HR, McNevin N, Ryan DH (August 2003). "Effects of tyrosine, phentermine, caffeine D-amphetamine, and placebo on cognitive and motor performance deficits during sleep deprivation". Nutritional Neuroscience 6 (4): 237–46. doi:10.1080/1028415031000120552. PMID 12887140.
- ↑ Neri DF, Wiegmann D, Stanny RR, Shappell SA, McCardie A, McKay DL (April 1995). "The effects of tyrosine on cognitive performance during extended wakefulness". Aviation, Space, and Environmental Medicine 66 (4): 313–9. PMID 7794222.
- ↑ "Dietary tyrosine suppresses the rise in plasma corticosterone following acute stress in rats". Life Sciences 37 (23): 2157–63. December 1985. doi:10.1016/0024-3205(85)90566-1. PMID 4068899.
- ↑ "Tyrosine improves cognitive performance and reduces blood pressure in cadets after one week of a combat training course". Brain Research Bulletin 48 (2): 203–9. January 1999. doi:10.1016/S0361-9230(98)00163-4. PMID 10230711.
- ↑ Mahoney CR, Castellani J, Kramer FM, Young A, Lieberman HR (November 2007). "Tyrosine supplementation mitigates working memory decrements during cold exposure". Physiology & Behavior 92 (4): 575–82. doi:10.1016/j.physbeh.2007.05.003. PMID 17585971. https://zenodo.org/record/1259309.
- ↑ "Effects of L-tyrosine and carbohydrate ingestion on endurance exercise performance". Journal of Applied Physiology 93 (5): 1590–7. November 2002. doi:10.1152/japplphysiol.00625.2001. PMID 12381742.
- ↑ Strüder HK, Hollmann W, Platen P, Donike M, Gotzmann A, Weber K (April 1998). "Influence of paroxetine, branched-chain amino acids and tyrosine on neuroendocrine system responses and fatigue in humans". Hormone and Metabolic Research 30 (4): 188–94. doi:10.1055/s-2007-978864. PMID 9623632.
- ↑ Thomas JR, Lockwood PA, Singh A, Deuster PA (November 1999). "Tyrosine improves working memory in a multitasking environment". Pharmacology, Biochemistry, and Behavior 64 (3): 495–500. doi:10.1016/S0091-3057(99)00094-5. PMID 10548261.
- ↑ "Efficacy of coenzyme Q10 in patients with cardiac failure: a meta-analysis of clinical trials". BMC Cardiovascular Disorders 17 (1): 196. July 2017. doi:10.1186/s12872-017-0628-9. PMID 28738783. PMC 5525208. //www.ncbi.nlm.nih.gov/pmc/articles/PMC5525208/.
- ↑ "Coenzyme Q10: a therapy for hypertension and statin-induced myalgia?". Cleveland Clinic Journal of Medicine 77 (7): 435–42. July 2010. doi:10.3949/ccjm.77a.09078. PMID 20601617.
- ↑ "Canadian Headache Society guideline for migraine prophylaxis". The Canadian Journal of Neurological Sciences 39 (2 Suppl 2): S1-59. March 2012. PMID 22683887.
- ↑ 59.0 59.1 59.2 59.3 59.4 "Coenzyme Q10 contents in foods and fortification strategies". Critical Reviews in Food Science and Nutrition 50 (4): 269–80. April 2010. doi:10.1080/10408390902773037. PMID 20301015.
- ↑ Cesar G. Fraga. Plant Phenolics and Human Health–Biochemistry, Nutrition and Pharmacology. John Wiley & Sons. p.9
- ↑ SemperBlotto (28 March 2006). "flavonoid". San Francisco, California: Wikimedia Foundation, Inc. Retrieved 8 August 2021.
{{cite web}}:|author=has generic name (help) - ↑ -sche (2 August 2020). "flavonoid". San Francisco, California: Wikimedia Foundation, Inc. Retrieved 8 August 2021.
{{cite web}}:|author=has generic name (help) - ↑ SemperBlotto (28 March 2006). "flavone". San Francisco, California: Wikimedia Foundation, Inc. Retrieved 8 August 2021.
{{cite web}}:|author=has generic name (help) - ↑ The compound in the Mediterranean diet that makes cancer cells 'mortal' Emily Caldwell, Medical Express, May 20, 2013.
- ↑ "Curcumin and Apigenin - novel and promising therapeutics against chronic neuroinflammation in Alzheimer's disease". Neural Regeneration Research 10 (8): 1181–5. August 2015. doi:10.4103/1673-5374.162686. PMID 26487830. PMC 4590215. //www.ncbi.nlm.nih.gov/pmc/articles/PMC4590215/.
- ↑ "Plant flavone apigenin: An emerging anticancer agent". Current Pharmacology Reports 3 (6): 423–446. 2017. doi:10.1007/s40495-017-0113-2. PMID 29399439. PMC 5791748. //www.ncbi.nlm.nih.gov/pmc/articles/PMC5791748/.
- ↑ 67.0 67.1 67.2 Delage, PhD, Barbara (November 2015). "Flavonoids". Linus Pauling Institute, Oregon State University, Corvallis, Oregon. Retrieved 2021-01-26.
- ↑ 68.0 68.1 Mann, John (1992). Secondary Metabolism (2nd ed.). Oxford, UK: Oxford University Press. pp. 279–280. ISBN 978-0-19-855529-2. https://archive.org/details/secondarymetabol00mann/page/279.
- ↑ Yong Lin; Ranxin Shi; Xia Wang; Han-Ming Shen (2008). "Luteolin, a flavonoid with potentials for cancer prevention and therapy". Curr Cancer Drug Targets 42 (7): 634–646. doi:10.2174/156800908786241050.
- ↑ Ayhan Ulubelen; M. Miski; P. Neuman; T. J. Mabry (1979). "Flavonoids of Salvia tomentosa (Labiatae)". Journal of Natural Products 42 (4): 261–3. doi:10.1021/np50003a002.
- ↑ Kayoko Shimoi; Hisae Okada; Michiyo Furugori; Toshinao Goda; Sachiko Takase; Masayuki Suzuki; Yukihiko Hara; Hiroyo Yamamoto et al. (1998). "Intestinal absorption of luteolin and luteolin 7-O-[beta]-glucoside in rats and humans". FEBS Letters 438 (3): 220–4. doi:10.1016/S0014-5793(98)01304-0. PMID 9827549.
- ↑ López-Lázaro M. (2009). "Distribution and biological activities of the flavonoid luteolin". Mini Rev Med Chem 9 (1): 31–59. doi:10.2174/138955709787001712. PMID 19149659.
- ↑ Lee, D; Cuendet, M; Vigo, JS; Graham, JG; Cabieses, F; Fong, HH; Pezzuto, JM; Kinghorn, AD (2001). "A novel cyclooxygenase-inhibitory stilbenolignan from the seeds of Aiphanes aculeata". Organic Letters 3 (14): 2169–71. doi:10.1021/ol015985j. PMID 11440571.
- ↑ Ceccarelli N., Curadi M., Picciarelli P., Martelloni L., Sbrana C., Giovannetti M. "Globe artichoke as a functional food" Mediterranean Journal of Nutrition and Metabolism 2010 3:3 (197–201)
- ↑ Panizzi, Luigi; Scarpati, Maria Luisa (1954). "Constitution of Cynarine, the Active Principle of the Artichoke". Nature 174 (4440): 1062–3. doi:10.1038/1741062a0. PMID 13214078.
- ↑ 76.0 76.1 Stryer L; Berg JM; Tymoczko JL (2002). Biochemistry (5th ed.). San Francisco: W.H. Freeman. ISBN 0-7167-4955-6. https://www.ncbi.nlm.nih.gov/books/NBK21154/.
- ↑ Nomenclature Committee. "Classification and Nomenclature of Enzymes by the Reactions they Catalyse". International Union of Biochemistry and Molecular Biology (NC-IUBMB). School of Biological and Chemical Sciences, Queen Mary, University of London. Retrieved 6 March 2015.
- ↑ Nomenclature Committee. "EC 2.7.1.1". International Union of Biochemistry and Molecular Biology (NC-IUBMB). School of Biological and Chemical Sciences, Queen Mary, University of London. Retrieved 6 March 2015.
- ↑ Mulder NJ (2007-09-28). "Protein Family Databases". eLS. Chichester, UK: John Wiley & Sons, Ltd. pp. a0003058.pub2. doi:10.1002/9780470015902.a0003058.pub2. ISBN 978-0-470-01617-6.
- ↑ Fraser PD; Linden H; Sandmann G (May 1993). "Purification and reactivation of recombinant Synechococcus phytoene desaturase from an overexpressing strain of Escherichia coli". The Biochemical Journal 291 ( Pt 3) (3): 687–92. doi:10.1042/bj2910687. PMID 8489496.
- ↑ Schneider C; Böger P; Sandmann G (July 1997). "Phytoene desaturase: heterologous expression in an active state, purification, and biochemical properties". Protein Expression and Purification 10 (2): 175–9. doi:10.1006/prep.1997.0730. PMID 9226712.
- ↑ Breitenbach J; Sandmann G (March 2005). "zeta-Carotene cis isomers as products and substrates in the plant poly-cis carotenoid biosynthetic pathway to lycopene". Planta 220 (5): 785–93. doi:10.1007/s00425-004-1395-2. PMID 15503129.
- ↑ Moise AR; Al-Babili S; Wurtzel ET (31 October 2013). "Mechanistic aspects of carotenoid biosynthesis". Chemical Reviews 114 (1): 164–193. doi:10.1021/cr400106y. PMID 24175570.
- ↑ SemperBlotto (8 March 2005). "fatty acid". San Francisco, California: Wikimedia Foundation, Inc. Retrieved 17 July 2021.
{{cite web}}:|author=has generic name (help) - ↑ SemperBlotto (14 May 2005). "fatty acid". San Francisco, California: Wikimedia Foundation, Inc. Retrieved 17 July 2021.
{{cite web}}:|author=has generic name (help) - ↑ SemperBlotto (5 June 2005). "fatty acid". San Francisco, California: Wikimedia Foundation, Inc. Retrieved 17 July 2021.
{{cite web}}:|author=has generic name (help) - ↑ Katrin Schütz, Reinhold Carle; Andreas Schieber (2006). "Taraxacum—a review on its phytochemical and pharmacological profile". Journal of Ethnopharmacology 107 (3): 313–323. doi:10.1016/j.jep.2006.07.021. PMID 16950583.
- ↑ "Carotenoids". Micronutrient Information Center, Linus Pauling Institute, Oregon State University. 1 July 2016. Retrieved 27 June 2019.
- ↑ Blumberg, JB; Camesano, TA; Cassidy, A; Kris-Etherton, P; Howell, A; Manach, C; Ostertag, LM; Sies, H et al. (2013). "Cranberries and their bioactive constituents in human health.". Advances in Nutrition 4 (6): 618–32. doi:10.3945/an.113.004473. ISSN 2161-8313. PMID 24228191. PMC 3823508. //www.ncbi.nlm.nih.gov/pmc/articles/PMC3823508/.
- ↑ "Analysis of Conformational, Structural, Magnetic, and Electronic Properties Related to Antioxidant Activity: Revisiting Flavan, Anthocyanidin, Flavanone, Flavonol, Isoflavone, Flavone, and Flavan-3-ol". ACS Omega 6 (13): 8908–8918. April 2021. doi:10.1021/acsomega.0c06156. PMID 33842761.
- ↑ IUPAC Compendium of Chemical Terminology (2nd ed.). Oxford: Blackwell Scientific. 1997. doi:10.1351/goldbook.F02424. ISBN 978-0-9678550-9-7.
- ↑ "Flavonoids (isoflavonoids and neoflavonoids)". The Gold Book. 2009. doi:10.1351/goldbook. ISBN 978-0-9678550-9-7. http://goldbook.iupac.org. Retrieved 16 September 2012.
- ↑ "Chaste tree". Drugs.com. 9 October 2017. Retrieved 20 August 2019.
- ↑ 94.0 94.1 Hoberg, Eva; Meier, Beat; Sticher, Otto (2000). "An analytical high performance liquid chromatographic method for the determination of agnuside and p-hydroxybenzoic acid contents in Agni-casti fructus". Phytochemical Analysis 11 (5): 327–329. doi:10.1002/1099-1565(200009/10)11:5<327::AID-PCA523>3.0.CO;2-0.
- ↑ Hajdú, Zsuzsanna; Hohmann, Judit; Forgo, Peter; Martinek, Tamás; Dervarics, Máté; Zupkó, István; Falkay, György; Cossuta, Daniel et al. (2007). "Diterpenoids and flavonoids from the fruits of Vitex agnus-castus and antioxidant activity of the fruit extracts and their constituents". Phytotherapy Research 21 (4): 391–394. doi:10.1002/ptr.2021. PMID 17262892.
- ↑ 96.0 96.1 Marder M, Viola H, Wasowski C, Fernández S, Medina JH, Paladini AC (2003). "6-methylapigenin and hesperidin: new valeriana flavonoids with activity on the CNS". Pharmacol Biochem Behav 75 (3): 537–45. doi:10.1016/S0091-3057(03)00121-7. PMID 12895671.
- ↑ Fernández S, Wasowski C, Paladini AC, Marder M (2004). "Sedative and sleep-enhancing properties of linarin, a flavonoid-isolated from Valeriana officinalis". Pharmacol Biochem Behav 77 (2): 399–404. doi:10.1016/j.pbb.2003.12.003. PMID 14751470.
- ↑ SemperBlotto (6 April 2008). "flavonol". San Francisco, California: Wikimedia Foundation, Inc. Retrieved 8 August 2021.
{{cite web}}:|author=has generic name (help) - ↑ Zapesochnaya, G. G.; Kurkin, V. A. (1983). "The flavonoids of the rhizomes ofRhodiola rosea. II. A flavonolignan and glycosides of herbacetin". Chemistry of Natural Compounds 19: 21–29. doi:10.1007/BF00579955.
- ↑ María Luisa Mateos-Martín, Elisabet Fuguet, Carmen Quero, Jara Pérez-Jiménez, Josep Lluís Torres; Fuguet; Quero; Pérez-Jiménez; Torres (2012). "New identification of proanthocyanidins in cinnamon (Cinnamomum zeylanicum L.) using MALDI-TOF/TOF mass spectrometry". Analytical and Bioanalytical Chemistry 402 (3): 1327–1336. doi:10.1007/s00216-011-5557-3. PMID 22101466.
- ↑ Souquet, J; Cheynier, Véronique; Brossaud, Franck; Moutounet, Michel (1996). "Polymeric proanthocyanidins from grape skins". Phytochemistry 43 (2): 509–512. doi:10.1016/0031-9422(96)00301-9.
- ↑ "USDA Database for the Proanthocyanidin Content of Selected Foods – 2004" (PDF). USDA. 2004. Retrieved 24 April 2014.
- ↑ Vivas, N; Nonier, M; Pianet, I; Vivasdegaulejac, N; Fouquet, E (2006). "Proanthocyanidins from Quercus petraea and Q. robur heartwood: quantification and structures". Comptes Rendus Chimie 9: 120–126. doi:10.1016/j.crci.2005.09.001.
- ↑ "Chemical composition, antioxidant properties, and thermal stability of a phytochemical enriched oil from Acai (Euterpe oleracea Mart.)". J Agric Food Chem 56 (12): 4631–6. June 2008. doi:10.1021/jf800161u. PMID 18522407.
- ↑ Hammerstone, John F.; Lazarus, Sheryl A.; Schmitz, Harold H. (August 2000). "Procyanidin content and variation in some commonly consumed foods". The Journal of Nutrition 130 (8S Suppl): 2086S–92S. doi:10.1093/jn/130.8.2086S. PMID 10917927. "Figure 5"
- ↑ Rohdewald, P (2002). "A review of the French maritime pine bark extract (Pycnogenol), a herbal medication with a diverse clinical pharmacology". International Journal of Clinical Pharmacology and Therapeutics 40 (4): 158–68. doi:10.5414/cpp40158. PMID 11996210.
- ↑ Hatano, T; Miyatake, H; Natsume, M; Osakabe, N; Takizawa, T; Ito, H; Yoshida, T (2002). "Proanthocyanidin glycosides and related polyphenols from cacao liquor and their antioxidant effects". Phytochemistry 59 (7): 749–58. doi:10.1016/S0031-9422(02)00051-1. PMID 11909632.
- ↑ Merghem, R.; Jay, M.; Brun, N.; Voirin, B. (2004). "Qualitative analysis and HPLC isolation and identification of procyanidins fromvicia faba". Phytochemical Analysis 15 (2): 95–99. doi:10.1002/pca.731. PMID 15116939.
- ↑ Van Der Poel, A. F. B.; Dellaert, L. M. W.; Van Norel, A.; Helsper, J. P. F. G. (2007). "The digestibility in piglets of faba bean (Vicia faba L.) as affected by breeding towards the absence of condensed tannins". British Journal of Nutrition 68 (3): 793–800. doi:10.1079/BJN19920134. PMID 1493141.
- ↑ Griffiths, D. W. (1981). "The polyphenolic content and enzyme inhibitory activity of testas from bean (Vicia faba) and pea (Pisum spp.) varieties". Journal of the Science of Food and Agriculture 32 (8): 797–804. doi:10.1002/jsfa.2740320808.
- ↑ Qa’Dan, F.; Petereit, F.; Mansoor, K.; Nahrstedt, A. (2006). "Antioxidant oligomeric proanthocyanidins fromCistus salvifolius". Natural Product Research 20 (13): 1216–1224. doi:10.1080/14786410600899225. PMID 17127512.
- ↑ "Bioactivity of dietary polyphenols: The role of metabolites". Critical Reviews in Food Science and Nutrition 60 (4): 626–659. 2020. doi:10.1080/10408398.2018.1546669. PMID 30614249.
- ↑ Aizpurua-Olaizola, Oier; Ormazabal, Markel; Vallejo, Asier; Olivares, Maitane; Navarro, Patricia; Etxebarria, Nestor; Usobiaga, Aresatz (2015-01-01). "Optimization of Supercritical Fluid Consecutive Extractions of Fatty Acids and Polyphenols from Vitis Vinifera Grape Wastes". Journal of Food Science 80 (1): E101–E107. doi:10.1111/1750-3841.12715. ISSN 1750-3841. PMID 25471637.
- ↑ 114.0 114.1 114.2 "Flavonoids". Micronutrient Information Center, Linus Pauling Institute, Oregon State University, Corvallis, Oregon. November 2015. Retrieved 25 February 2018.
- ↑ Morling, J. R; Yeoh, S. E; Kolbach, D. N (November 2018). "Rutosides for treatment of post-thrombotic syndrome". Cochrane Database of Systematic Reviews 11 (11): CD005625. doi:10.1002/14651858.CD005625.pub4. PMID 30406640.
- ↑ Martinez-Zapata, M. J; Vernooij, R. W; Uriona Tuma, S. M; Stein, A. T; Moreno, R. M; Vargas, E; Capellà, D; Bonfill Cosp, X (2016). "Phlebotonics for venous insufficiency". Cochrane Database of Systematic Reviews 4: CD003229. doi:10.1002/14651858.CD003229.pub3. PMID 27048768. https://www.ncbi.nlm.nih.gov/pubmed/33141449.
- ↑ Struijs, K.; Vincken, J. P.; Verhoef, R.; Van Oostveen-Van Casteren, W. H. M.; Voragen, A. G. J.; Gruppen, H. (2007). "The flavonoid herbacetin diglucoside as a constituent of the lignan macromolecule from flaxseed hulls". Phytochemistry 68 (8): 1227–1235. doi:10.1016/j.phytochem.2006.10.022. PMID 17141814.
- ↑ Li, T.; Zhang, H. (2008). "Identification and Comparative Determination of Rhodionin in Traditional Tibetan Medicinal Plants of Fourteen Rhodiola Species by High-Performance Liquid Chromatography-Photodiode Array Detection and Electrospray Ionization-Mass Spectrometry". Chemical & Pharmaceutical Bulletin 56 (6): 807–14. doi:10.1248/cpb.56.807. PMID 18520085.
- ↑ "Antidiabetes and Anti-obesity Activity of Lagerstroemia speciosa"
- ↑ Asiatic Acid, Corosolic Acid, and Maslinic Acid Interfere with Intracellular Trafficking and N-Linked Glycosylation of Intercellular Adhesion Molecule-1. doi:10.1248/bpb.b18-00276.
- ↑ Tanaka, Yoshitaka; Van Ke, Nguyen (2007). Edible Wild Plants of Vietnam: The Bountiful Garden. Thailand: Orchid Press. p. 90. ISBN 978-9745240896.
- ↑ "Lagerstroemia speciosa (L.) Pers. pride of India." PLANTS Profile, United States Department of Agriculture / Natural Resources Conservation Service. Retrieved 2008-07-15.
- ↑ 123.0 123.1 123.2 123.3 123.4 123.5 123.6 123.7 123.8 "Lignans". Micronutrient Information Center, Linus Pauling Institute, Oregon State University. 2010. Retrieved 31 July 2017.
- ↑ From lign- (Latin, "wood") + -an (chemical suffix).
- ↑ Korkina, L; Kostyuk, V; De Luca, C; Pastore, S (2011). "Plant phenylpropanoids as emerging anti-inflammatory agents". Mini Reviews in Medicinal Chemistry 11 (10): 823–35. doi:10.2174/138955711796575489. PMID 21762105.
- ↑ Saleem, Muhammad; Kim, Hyoung Ja; Ali, Muhammad Shaiq; Lee, Yong Sup (2005). "An update on bioactive plant lignans". Natural Product Reports 22 (6): 696–716. doi:10.1039/B514045P. PMID 16311631.
- ↑ Umezawa, Toshiaki (2003). "Diversity in lignan biosynthesis". Phytochemistry Reviews 2 (3): 371–90. doi:10.1023/B:PHYT.0000045487.02836.32.
- ↑ Adlercreutz, Herman (2007). "Lignans and Human Health". Critical Reviews in Clinical Laboratory Sciences 44 (5–6): 483–525. doi:10.1080/10408360701612942. PMID 17943494.
- ↑ Heinonen, S; Nurmi, T; Liukkonen, K; Poutanen, K; Wähälä, K; Deyama, T; Nishibe, S; Adlercreutz, H (2001). "In vitro metabolism of plant lignans: New precursors of mammalian lignans enterolactone and enterodiol". Journal of Agricultural and Food Chemistry 49 (7): 3178–86. doi:10.1021/jf010038a. PMID 11453749.
- ↑ 130.0 130.1 Landete, José (2012). "Plant and mammalian lignans: A review of source, intake, metabolism, intestinal bacteria and health". Food Research International 46 (1): 410–24. doi:10.1016/j.foodres.2011.12.023. https://www.sciencedirect.com/science/article/abs/pii/S0963996912000087.
- ↑ "Lignan contents of Dutch plant foods: a database including lariciresinol, pinoresinol, secoisolariciresinol and matairesinol". British Journal of Nutrition 93 (3): 393–402. 2005. doi:10.1079/BJN20051371. PMID 15877880.
- ↑ Han, H.; Jung, J.K.; Han, S.B.; Nam, S.Y.; Oh, K.W.; Hong, J.T. (2011). "Anxiolytic-like effects of 4-O-methylhonokiol isolated from magnolia officinalis through enhancement of GABAergic transmission and chloride influx". Journal of Medicinal Food 14 (7–8): 724–731. doi:10.1089/jmf.2010.1111. PMID 21501091.
- ↑ Kalman, D.S.; Feldman, S.; Feldman, R.; Schwartz, H.I.; Krieger, D.R.; Garrison, R. (2008). "Effect of a proprietary Magnolia and Phellodendron extract on stress levels in healthy women: A pilot, double-blind, placebo-controlled clinical trial". Nutrition Journal 7 (1): 11. doi:10.1186/1475-2891-7-11. PMID 18426577. PMC 2359758. //www.ncbi.nlm.nih.gov/pmc/articles/PMC2359758/.
- ↑ Ma, L.; Chen, J.; Wang, X.; Liang, X.; Luo, Y.; Zhu, W.; Wang, T.; Peng, M. et al. (2011). "Structural modification of honokiol, a biphenyl occurring in magnolia officinalis: The evaluation of honokiol analogues as inhibitors of angiogenesis and for their cytotoxicity and structure-activity relationship". Journal of Medicinal Chemistry 54 (19): 6469–6481. doi:10.1021/jm200830u. PMID 21853991.
- ↑ Fried, L.E.; Arbiser, J.L. (2009). "Honokiol, a multifunctional antiangiogenic and antitumor agent". Antioxidants & Redox Signaling 11 (5): 1139–1148. doi:10.1089/ars.2009.2440. PMID 19203212.
- ↑ Hu J.; Chen L.-J.; Liu L.; Chen X.; Chen P.; Yang G.-L.; Hou W.-L.; Tang M.-H. et al. (2008). "Liposomal honokiol, a potent anti-angiogenesis agent, in combination with radiotherapy produces a synergistic antitumor efficacy without increasing toxicity". Experimental & Molecular Medicine 40 (6): 617–628. doi:10.3858/emm.2008.40.6.617. PMID 19116447.
- ↑ "Therapeutic applications of compounds in the Magnolia family". Pharmacol. Ther. 130 (2): 157–176. 2011. doi:10.1016/j.pharmthera.2011.01.010. PMID 21277893.
- ↑ Fakhrudin, N.; Ladurner, A.; Atanasov, A.G.; Heiss, E.H.; Baumgartner, L.; Markt, P.; Schuster, D.; Ellmerer, E.P. et al. (April 2010). "Computer-aided discovery, validation, and mechanistic characterization of novel neolignan activators of peroxisome proliferator-activated receptor gamma". Mol. Pharmacol. 77 (4): 559–66. doi:10.1124/mol.109.062141. PMID 20064974.
- ↑ Atanasov AG, Wang JN, Gu SP, Bu J, Kramer MP, Baumgartner L, Fakhrudin N, Ladurner A, Malainer C, Vuorinen A, Noha SM, Schwaiger S, Rollinger JM, Schuster D, Stuppner H, Dirsch VM, Heiss EH (October 2013). "Honokiol: A non-adipogenic PPARγ agonist from nature". Biochimica et Biophysica Acta (BBA) - General Subjects 1830 (10): 4813–4819. doi:10.1016/j.bbagen.2013.06.021. PMID 23811337. PMC 3790966. //www.ncbi.nlm.nih.gov/pmc/articles/PMC3790966/.
- ↑ Lee, Young-Jung; Lee, Yoot Mo; Lee, Chong-Kil; Jung, Jae Kyung; Han, Sang Bae; Hong, Jin Tae (2011). "Therapeutic applications of compounds in the Magnolia family". Pharmacology & Therapeutics 130 (2): 157–76. doi:10.1016/j.pharmthera.2011.01.010. PMID 21277893.
- ↑ Ai, Jinglu; Wang, Xiaomei; Nielsen, Mogens (2001). "Honokiol and Magnolol Selectively Interact with GABAA Receptor Subtypes in vitro". Pharmacology 63 (1): 34–41. doi:10.1159/000056110. PMID 11408830.
- ↑ Bang, Kyu Ho; Kim, Yoon Kwan; Min, Byung Sun; Na, Min Kyun; Rhee, Young Ha; Lee, Jong Pill; Bae, Ki Hwan (2000). "Antifungal activity of magnolol and honokiol". Archives of Pharmacal Research 23 (1): 46–9. doi:10.1007/BF02976465. PMID 10728656.
- ↑ Kwak, Eun Jung; Lee, Young Soon; Choi, Eun Mi (2012). "Effect of Magnolol on the Function of Osteoblastic MC3T3-E1 Cells". Mediators of Inflammation 2012: 1–7. doi:10.1155/2012/829650. PMID 22474400. PMC 3306956. //www.ncbi.nlm.nih.gov/pmc/articles/PMC3306956/.
- ↑ Lu, Sheng-Hua; Huang, Ren-Yeong; Chou, Tz-Chong (2013). "Magnolol Ameliorates Ligature-Induced Periodontitis in Rats and Osteoclastogenesis: In Vivo and in Vitro Study". Evidence-Based Complementary and Alternative Medicine 2013: 1–12. doi:10.1155/2013/634095. PMID 23573141. PMC 3618931. //www.ncbi.nlm.nih.gov/pmc/articles/PMC3618931/.
- ↑ Fuchs, A; Baur, R; Schoeder, C; Sigel, E; Müller, CE (December 15, 2014). "Structural analogues of the natural products magnolol and honokiol as potent allosteric potentiators of GABAA receptors.". Bioorg Med Chem 22 (24): 6908–17. doi:10.1016/j.bmc.2014.10.027. PMID 25456080.
- ↑ Dreier D, Latkolik S, Rycek L, Schnürch M, Dymáková A, Atanasov AG, Ladurner A, Heiss EH, Stuppner H, Schuster D, Mihovilovic MD, Dirsch VM. Linked magnolol dimer as a selective PPARγ agonist - Structure-based rational design, synthesis, and bioactivity evaluation. Sci Rep. 2017 Oct 20;7(1):13002. doi: 10.1038/s41598-017-12628-5.
- ↑ Barros J, Serrani-Yarce JC, Chen F, Baxter D, Venables BJ, Dixon RA (2016). "Role of bifunctional ammonia-lyase in grass cell wall biosynthesis". Nat. Plants 2: 16050. doi:10.1038/nplants.2016.50. PMID 27255834.
- ↑ 148.0 148.1 Vogt, T. (2010). "Phenylpropanoid Biosynthesis". Molecular Plant 3: 2–20. doi:10.1093/mp/ssp106. PMID 20035037.
- ↑ "Effect of resveratrol on blood pressure: A systematic review and meta-analysis of randomized, controlled, clinical trials". Critical Reviews in Food Science and Nutrition 58 (2): 1605–1618. January 2018. doi:10.1080/10408398.2017.1422480. PMID 29359958.
- ↑ Liu Y, Ma W, Zhang P, He S, Huang D; Ma; Zhang; He; Huang (March 2014). "Effect of resveratrol on blood pressure: A meta-analysis of randomized controlled trials". Clinical Nutrition 34 (1): 27–34. doi:10.1016/j.clnu.2014.03.009. PMID 24731650.
- ↑ Zhu, Xiangyun; Wu, Chunhua; Qiu, Shanhu; Yuan, Xuelu; Li, Ling (22 September 2017). "Effects of resveratrol on glucose control and insulin sensitivity in subjects with type 2 diabetes: systematic review and meta-analysis". Nutrition & Metabolism 14 (1): 60. doi:10.1186/s12986-017-0217-z. ISSN 1743-7075. PMID 29018489. PMC 5610395. //www.ncbi.nlm.nih.gov/pmc/articles/PMC5610395/.
- ↑ Mousavi, S. M.; Milajerdi, A.; Sheikhi, A.; Kord‐Varkaneh, H.; Feinle‐Bisset, C.; Larijani, B.; Esmaillzadeh, A. (2019). "Resveratrol supplementation significantly influences obesity measures: a systematic review and dose–response meta-analysis of randomized controlled trials". Obesity Reviews 20 (3): 487–498. doi:10.1111/obr.12775. PMID 30515938.
- ↑ Asgary, Sedigheh; Karimi, Raheleh; Momtaz, Saeideh; Naseri, Rozita; Farzaei, Mohammad Hosein (1 June 2019). "Effect of resveratrol on metabolic syndrome components: A systematic review and meta-analysis". Reviews in Endocrine and Metabolic Disorders 20 (2): 173–186. doi:10.1007/s11154-019-09494-z. PMID 31065943.
- ↑ Koushki, Mehdi; Dashatan, Nasrin Amiri; Meshkani, Reza (July 2018). "Effect of Resveratrol Supplementation on Inflammatory Markers: A Systematic Review and Meta-analysis of Randomized Controlled Trials". Clinical Therapeutics 40 (7): 1180–1192.e5. doi:10.1016/j.clinthera.2018.05.015. PMID 30017172.
- ↑ BSBI List 2007, Botanical Society of Britain and Ireland, retrieved 2015-06-26
- ↑ Gaid, Mariam; Biedermann, Eline; Füller, Jendrik; Haas, Paul; Behrends, Sönke; Krull, Rainer; Scholl, Stephan; Wittstock, Ute et al. (2018). "Biotechnological production of hyperforin for pharmaceutical formulation". Eur. J. Pharm. Biopharm. 126: 10–26. doi:10.1016/j.ejpb.2017.03.024. PMID 28377273.
- ↑ Ciochina, Roxana; Grossman, Robert B. (2006). "Polycyclic Polyprenylated Acylphloroglucinols". Chem. Rev. 106 (9): 3963–3986. doi:10.1021/cr0500582. PMID 16967926.
- ↑ 158.0 158.1 Sparling B, Moebius D, Shair M (December 2012). "Enantioselective Total Synthesis of Hyperforin". J Am Chem Soc 135 (2): 644–7. doi:10.1021/ja312150d. PMID 23270309. http://nrs.harvard.edu/urn-3:HUL.InstRepos:12563735.
- ↑ Bystrov NS; Gupta ShR; Dobrynin VN; Kolosov MN; Chernov BK (January 1976). "[Structure of the antibiotic hyperforin]". Doklady Akademii Nauk SSSR 226 (1): 88–90. PMID 1248360.
- ↑ "[The structure of hyperforin]". Tetrahedron Letters 16 (32): 2791–2794. 1975. doi:10.1016/S0040-4039(00)75241-5.
- ↑ "Catalytic Asymmetric Total Synthesis of ent-Hyperforin". Angew Chem Int Ed 49 (6): 1103–6. February 2010. doi:10.1002/anie.200906678. PMID 20063336.
- ↑ Liu, F; Pan, C; Drumm, P; Ang, CY (February 2005). "Liquid chromatography-mass spectrometry studies of St. John's wort methanol extraction: active constituents and their transformation". Journal of Pharmaceutical and Biomedical Analysis 37 (2): 303–12. doi:10.1016/j.jpba.2004.10.034. PMID 15708671.
- ↑ Vajs, V.; Vugdelija, S.; Trifunović, S.; Karadžić, I.; Juranić, N.; MacUra, S.; Milosavljević, S. (2003). "Further degradation product of hyperforin from Hypericum perforatum (St. John's Wort)". Fitoterapia 74 (5): 439–444. doi:10.1016/S0367-326X(03)00114-X. PMID 12837358.
- ↑ Verotta, Luisella; Appendino, Giovanni; Belloro, Emanuela; Jakupovic, Jasmin; Bombardelli, Ezio (1999). "Furohyperforin, a Prenylated Phloroglucinol from St. John's Wort (Hypericumperforatum)". J. Nat. Prod. 62 (5): 770–772. doi:10.1021/np980470v. PMID 10346967.
- ↑ 165.0 165.1 Pushpa Khanna, S. C. Jain, A. Panagariya, and V. P. Dixit (November 1, 1981). "Hypoglycemic Activity of Polypeptide-p From a Plant Source". Journal of Natural Products 44 (6): 648–655. doi:10.1021/np50018a002. https://pubs.acs.org/doi/pdf/10.1021/np50018a002. Retrieved 8 July 2021.
- ↑ Sahu, N. P. (2008). Banerjee. ed. Steroidal Saponins, In: Fortschritte der Chemie organischer Naturstoffe / Progress in the Chemistry of Organic Natural Products. Springer Vienna. pp. 45–141. doi:10.1007/978-3-211-74019-4_2. ISBN 9783211740187.
- ↑ Molham Al-Habori and Amala Raman (18 December 1998). "Antidiabetic and hypocholesterolaemic effects of fenugreek". Phytotherapy Research 12 (4): 233-242. doi:10.1002/(SICI)1099-1573(199806)12:4<233::AID-PTR294>3.0.CO;2-V. https://onlinelibrary.wiley.com/doi/abs/10.1002/(SICI)1099-1573(199806)12:4%3C233::AID-PTR294%3E3.0.CO;2-V. Retrieved 5 August 2021.
- ↑ S.Mirunalini; Shahira (2011). "Novel Effects of Diosgenin – A Plant Derived Steroid; A Review" (PDF). PhOL - PharmacologyOnLine.
- ↑ A. Anbarasi (2016). Safety and Pharmacological Profile of Pattai vallathagi. Chennai, India: National Institute of Siddha. pp. 19 of 122. http://repository-tnmgrmu.ac.in/2455/7/320218316anbarasi.pdf. Retrieved 5 August 2021.
- ↑ Ramelet, Albert-Adrien. "Venoactive Drugs". Sclerotherapy: treatment of varicose and telangiectatic leg veins (6th ed.). Elsevier Science Health Science. pp. 426–434. ISBN 978-0-323-37726-3.
- ↑ Goldman, Mitchel P. (2016). Sclerotherapy : treatment of varicose and telangiectatic leg veins (6th ed.). Amsterdam: Elsevier Science Health Science. ISBN 978-0-323-37727-0.
- ↑ 172.0 172.1 172.2 Pittler, Max H.; Ernst, Edzard (2012-11-14). "Horse chestnut seed extract for chronic venous insufficiency". The Cochrane Database of Systematic Reviews 11: CD003230. doi:10.1002/14651858.CD003230.pub4. PMID 23152216.
- ↑ 173.0 173.1 Sirtori CR (September 2001). "Aescin: pharmacology, pharmacokinetics and therapeutic profile". Pharmacol. Res. 44 (3): 183–193. doi:10.1006/phrs.2001.0847. PMID 11529685.
- ↑ "Endothelium protectant and contractile effects of the antivaricose principle escin in rat aorta". Vascul. Pharmacol. 47 (1): 68–73. July 2007. doi:10.1016/j.vph.2007.04.003. PMID 17512261.
- ↑ "The mode of action of aescin and the release of prostaglandins". Prostaglandins 14 (2): 241–249. August 1977. doi:10.1016/0090-6980(77)90169-1. PMID 897216.
- ↑ Liu, X; Liu, F; Yue, R; Li, Y; Zhang, J; Wang, S; Zhang, S; Wang, R et al. (2013). "The antidepressant-like effect of bacopaside I: Possible involvement of the oxidative stress system and the noradrenergic system". Pharmacology Biochemistry and Behavior 110: 224–30. doi:10.1016/j.pbb.2013.07.007. PMID 23872136.
- ↑ Chakravarty, AK; Sarkar, T; Masuda, K; Shiojima, K; Nakane, T; Kawahara, N (2001). "Bacopaside I and II: Two pseudojujubogenin glycosides from Bacopa monniera". Phytochemistry 58 (4): 553–6. doi:10.1016/S0031-9422(01)00275-8. PMID 11576596.
- ↑ Hou, CC; Lin, SJ; Cheng, JT; Hsu, FL (2002). "Bacopaside III, bacopasaponin G, and bacopasides A, B, and C from Bacopa monniera". Journal of Natural Products 65 (12): 1759–63. doi:10.1021/np020238w. PMID 12502309.
- ↑ 179.0 179.1 179.2 Chakravarty, AK; Garai, S; Masuda, K; Nakane, T; Kawahara, N (2003). "Bacopasides III-V: Three new triterpenoid glycosides from Bacopa monniera". Chemical & Pharmaceutical Bulletin 51 (2): 215–7. doi:10.1248/cpb.51.215. PMID 12576661.
- ↑ 180.0 180.1 180.2 Zhou, Y; Shen, YH; Zhang, C; Su, J; Liu, RH; Zhang, WD (2007). "Triterpene saponins from Bacopa monnieri and their antidepressant effects in two mice models". Journal of Natural Products 70 (4): 652–5. doi:10.1021/np060470s. PMID 17343408.
- ↑ 181.0 181.1 181.2 Zhou, Y; Peng, L; Zhang, WD; Kong, DY (2009). "Effect of triterpenoid saponins from Bacopa monniera on scopolamine-induced memory impairment in mice". Planta Medica 75 (6): 568–74. doi:10.1055/s-0029-1185339. PMID 19214943.
- ↑ Jakobsson, Hugo; Farmaki, Katerina; Sakinis, Augustinas; Ehn, Olof; Johannsson, Gudmundur; Ragnarsson, Oskar (2018-05-15). "Adrenal venous sampling: the learning curve of a single interventionalist with 282 consecutive procedures". Diagnostic and Interventional Radiology 24 (2): 89–93. doi:10.5152/dir.2018.17397. PMID 29467114.
- ↑ "HerbalGram: Butcher's Broom". cms.herbalgram.org. Retrieved 2018-11-28.
- ↑ 184.0 184.1 Huang, Ya-Lin; Kou, Jun-Ping; Ma, Li; Song, Jia-Xi; Yu, Bo-Yang (October 2008). "Possible mechanism of the anti-inflammatory activity of ruscogenin: role of intercellular adhesion molecule-1 and nuclear factor-kappaB". Journal of Pharmacological Sciences 108 (2): 198–205. PMID 18946195.
- ↑ Bouskela, Eliete; Cyrino, Fatima Z. G. A.; Marcelon, Gilbert (August 1993). "Effects of Ruscus Extract on the Internal Diameter of Arterioles and Venules of the Hamster Cheek Pouch Microcirculation". Journal of Cardiovascular Pharmacology 22 (2): 221–224. doi:10.1097/00005344-199308000-00008.
- ↑ Helleboid S, Haug C, Lamottke K, et al. The Identification of Naturally Occurring Neoruscogenin as a Bioavailable, Potent, and High-Affinity Agonist of the Nuclear Receptor RORα (NR1F1). Journal of Biomolecular Screening. 2014;19(3):399-406. https://doi.org/10.1177/1087057113497095.
- ↑ Herrmann, Josef M.; Untergehrer, Monika; Jürgenliemk, Guido; Heilmann, Jörg; König, Burkhard (2014-04-08). "Synthesis of Phenyl-1-benzoxepinols Isolated from Butcher's Broom and Analogous Benzoxepines". European Journal of Organic Chemistry 2014 (15): 3170–3181. doi:10.1002/ejoc.201400004.
- ↑ Barbič, Matej; Schmidt, Thomas J.; Jürgenliemk, Guido (June 2012). "Novel Phenyl-1-benzoxepinols from Butcher's Broom (Rusci rhizoma)". Chemistry & Biodiversity 9 (6): 1077–1083. doi:10.1002/cbdv.201100158. PMID 22700226.
- ↑ Equinox (27 March 2009). "silymarin". San Francisco, California: Wikimedia Foundation, Inc. Retrieved 26 June 2021.
{{cite web}}:|author=has generic name (help) - ↑ "Milk Thistle and Prostate Cancer: Differential Effects of Pure Flavonolignans from Silybum marianum on Antiproliferative End Points in Human Prostate Carcinoma Cells". Cancer Research 65 (10): 4448–57. 2005. doi:10.1158/0008-5472.CAN-04-4662. PMID 15899838. http://cancerres.aacrjournals.org/content/canres/65/10/4448.full.pdf.
- ↑ "An updated systematic review with meta-analysis for the clinical evidence of silymarin". Forschende Komplementärmedizin 15 (1): 9–20. 2008. doi:10.1159/000113648. PMID 18334810. https://www.karger.com/Article/PDF/000113648. Retrieved 2010-12-14.
- ↑ 192.0 192.1 192.2 192.3 192.4 192.5 "Silychristin, a Flavonolignan Derived From the Milk Thistle, Is a Potent Inhibitor of the Thyroid Hormone Transporter MCT8". Endocrinology 157 (4): 1694–2301. 2016. doi:10.1210/en.2015-1933. PMID 26910310.
- ↑ "A review of the bioavailability and clinical efficacy of milk thistle phytosome: a silybin-phosphatidylcholine complex (Siliphos)". Alternative Medicine Review 10 (3): 193–203. 2005. PMID 16164374. https://web.archive.org/web/20110728045923/http://www.thorne.com/altmedrev/.fulltext/10/3/193.pdf. Retrieved 2010-12-14.
- ↑ "Pharmacokinetic studies on IdB 1016, a silybin-phosphatidylcholine complex, in healthy human subjects". Eur J Drug Metab Pharmacokinet 15 (4): 333–8. 1990. doi:10.1007/bf03190223. PMID 2088770.
- ↑ "Solid state mechanochemical activation of Silybum marianum dry extract with betacyclodextrins: Characterization and bioavailability of the coground systems". Journal of Pharmaceutical Sciences 98 (11): 4119–29. 2009. doi:10.1002/jps.21704. PMID 19226635.
- ↑ "Antioxidant properties of silybin glycosides". Phytotherapy Research 16 Suppl 1: S33–9. 2002. doi:10.1002/ptr.796. PMID 11933137.
- ↑ "Herbal modulation of P-glycoprotein". Drug Metabolism Reviews 36 (1): 57–104. 2004. doi:10.1081/DMR-120028427. PMID 15072439.
- ↑ "Drug-drug interactions of silymarin on the perspective of pharmacokinetics". Journal of Ethnopharmacology 121 (2): 185–93. 2009. doi:10.1016/j.jep.2008.10.036. PMID 19041708.
- ↑ "Allan-Herndon-Dudley syndrome | Genetic and Rare Diseases Information Center (GARD) – an NCATS Program". rarediseases.info.nih.gov. Retrieved 2021-05-06.
- ↑ Thomas W. Flaig; Daniel L. Gustafson; Lih-Jen Su; Joseph A. Zirrolli; Frances Crighton; Gail S. Harrison; A. Scott Pierson; Rajesh Agarwal et al. (2007). "A phase I and pharmacokinetic study of silybin-phytosome in prostate cancer patients". Investigational New Drugs 25 (2): 139–146. doi:10.1007/s10637-006-9019-2. PMID 17077998.
- ↑ "Pharmacology of Silymarin". Clinical Drug Investigation 22 (1): 51–65. 2002. doi:10.2165/00044011-200222010-00007. https://web.archive.org/web/20121027121930/http://adisonline.com/druginvestigation/Abstract/2002/22010/Pharmacology_of_Silymarin.7.aspx.
- ↑ "On the pharmacology and toxicology of silymarin, an antihepatotoxic active principle from Silybum marianum (L.) gaertn". Arzneimittelforschung 18 (6): 698–704. 1968. PMID 5755807.
- ↑ Rottapharm|Madaus. Media Communications Legalon®. Retrieved March 6, 2017.
- ↑ Bosch-Barrera, Joaquim; Sais, Elia; Cañete, Noemí; Marruecos, Jordi; Cuyàs, Elisabet; Izquierdo, Angel; Porta, Rut; Haro, Manel et al. (2016). "Response of brain metastasis from lung cancer patients to an oral nutraceutical product containing silibinin". Oncotarget 7 (22): 32006–32014. doi:10.18632/oncotarget.7900. PMID 26959886. PMC 5077992. //www.ncbi.nlm.nih.gov/pmc/articles/PMC5077992/.
- ↑ Singh, Rana P.; Agarwal, Rajesh (September 2009). "Cosmeceuticals and silibinin". Clinics in Dermatology 27 (5): 479–484. doi:10.1016/j.clindermatol.2009.05.012. PMID 19695480.
- ↑ Thakare, Vishnu N; Patil, Rajesh R; Oswal, Rajesh J; Dhakane, Valmik D; Aswar, Manoj K; Patel, Bhoomika M (February 2018). "Therapeutic potential of silymarin in chronic unpredictable mild stress induced depressive-like behavior in mice". Journal of Psychopharmacology 32 (2): 223–235. doi:10.1177/0269881117742666. PMID 29215318.
- ↑ Sarubbo, F.; Ramis, M. R.; Kienzer, C.; Aparicio, S.; Esteban, S.; Miralles, A.; Moranta, D. (March 2018). "Chronic Silymarin, Quercetin and Naringenin Treatments Increase Monoamines Synthesis and Hippocampal Sirt1 Levels Improving Cognition in Aged Rats". Journal of Neuroimmune Pharmacology 13 (1): 24–38. doi:10.1007/s11481-017-9759-0. PMID 28808887.
- ↑ Balouchi, Sima; Gharagozloo, Marjan; Esmaeil, Nafiseh; Mirmoghtadaei, Milad; Moayedi, Behjat (2014-08-01). "Serum levels of TGFβ, IL-10, IL-17, and IL-23 cytokines in β-thalassemia major patients: the impact of silymarin therapy". Immunopharmacology and Immunotoxicology 36 (4): 271–274. doi:10.3109/08923973.2014.926916. PMID 24945737.
- ↑ Esfahani, Behjat Al-Sadat Moayedi; Reisi, Nahid; Mirmoghtadaei, Milad (2015-03-04). "Evaluating the Safety and Efficacy of Silymarin in β-Thalassemia Patients: A Review". Hemoglobin 39 (2): 75–80. doi:10.3109/03630269.2014.1003224. PMID 25643967.
- ↑ "Substituted Pyrazinecarboxamides as Abiotic Elicitors of Flavolignan Production in Silybum marianum (L.) Gaertn Cultures in Vitro". Molecules 15 (1): 331–340. 2010. doi:10.3390/molecules15010331. PMID 20110894.
- ↑ Lv, Yongkun; Gao, Song; Xu, Sha; Du, Guocheng; Zhou, Jingwen; Chen, Jian (2017-11-11). "Spatial organization of silybin biosynthesis in milk thistle [Silybum marianum (L.) Gaertn"]. The Plant Journal 92 (6): 995–1004. doi:10.1111/tpj.13736. PMID 28990236. http://dx.doi.org/10.1111/tpj.13736.
- ↑ Prasad, Ram Raj; Paudel, Sandeep; Raina, Komal; Agarwal, Rajesh (May 2020). "Silibinin and non-melanoma skin cancers". Journal of Traditional and Complementary Medicine 10 (3): 236–244. doi:10.1016/j.jtcme.2020.02.003. PMID 32670818. http://dx.doi.org/10.1016/j.jtcme.2020.02.003.
- ↑ Barros, Jaime; Escamilla-Trevino, Luis; Song, Luhua; Rao, Xiaolan; Serrani-Yarce, Juan Carlos; Palacios, Maite Docampo; Engle, Nancy; Choudhury, Feroza K. et al. (2019-04-30). "4-Coumarate 3-hydroxylase in the lignin biosynthesis pathway is a cytosolic ascorbate peroxidase". Nature Communications 10 (1): 1994. doi:10.1038/s41467-019-10082-7. PMID 31040279. http://dx.doi.org/10.1038/s41467-019-10082-7.
- ↑ Barnes, J.; Anderson, L.A.; Phillipson, J.D. (1996). Herbal Medicines (3rd ed.). London, UK: Pharmaceutical Press. ISBN 978-0-85369-623-0. https://web.archive.org/web/20180701140306/http://file.zums.ac.ir/ebook/366-Herbal%20Medicines,%20Third%20edition-Joanne%20Barnes%20J.%20David%20Phillipson%20Linda%20A.%20Anderson-085369623.pdf. Retrieved 7 February 2015.
- ↑ "St. John's wort (Hypericum perforatum): a review of the current pharmacological, toxicological, and clinical literature". Psychopharmacology 153 (4): 402–414. February 2001. doi:10.1007/s002130000625. PMID 11243487. https://web.archive.org/web/20140611050347/http://sites.duke.edu/greesonlab/files/2011/07/Greeson_etal_2001_Psychopharm-St.-Johns-Wort.pdf. Retrieved 11 June 2014.
- ↑ 216.0 216.1 Mehta, Sweety (2012-12-18). "Pharmacognosy of St. John's Wort". Pharmaxchange.info. Retrieved 2014-02-16.
- ↑ "Quantitative phytochemical analyses of six hypericum species growing in slovenia". Planta Med. 65 (4): 388–90. 1999. doi:10.1055/s-2006-960798. PMID 17260265.
- ↑ "Identification of the major constituents of Hypericum perforatum by LC/SPE/NMR and/or LC/MS". Phytochemistry 68 (3): 383–93. 2007. doi:10.1016/j.phytochem.2006.11.026. PMID 17196625.
- ↑ Schwob I, Bessière JM, Viano J.Composition of the essential oils of Hypericum perforatum L. from southeastern France.C R Biol. 2002;325:781-5.
- ↑ Snitker S. et al. Effects of novel capsinoids treatment on fatness and energy metabolism in humans: possible pharmacogenetic implications. Am, J. Clin. Nutr. 89 45-50 (2009)
- ↑ Inoue N. et al., Biosci Biotechnol Biochem 71: 380-389, 2007.
- ↑ Ohnuki K. et al., Biosci Biotechnol Biochem 65:2033-2036, 2001(a).
- ↑ Sachiko Hachiya, Fuminori Kawabata, Koichiro Ohnuki, Naohiko Inoue, Hirotsugu Yoneda, Susumu Yazawa and Tohru Fushiki. "Effects of CH-19 Sweet, a Non-Pungent Cultivar of Red Pepper, on Sympathetic Nervous Activity, Body Temperature, Heart Rate, and Blood Pressure in Humans." Biosci. Biotechnol. Biochem. 71 (2007): 671-676.
- ↑ Ohnuki K. et al., J Nutr Sci Vitaminol 47:295-298, 2001.
- ↑ Ohnuki K. et al., Biosci Biotechnol Biochem 65:2735-2740, 2001.
- ↑ Reinbach H.C. et al., Clinical Nutrition 28: 260-265, 2009
Further reading
[edit | edit source]- Eric Braverman (1979). "Orthomolecular Medicine and Megavitamin Therapy: Future and Philosophy". Orthomolecular Psychiatry 8 (4): 265-72. http://www.orthomolecular.org/library/jom/1979/pdf/1979-v08n04-p265.pdf. Retrieved 2014-08-20.
External links
[edit | edit source]|
Learn more about Orthomolecular medicine |