 TIMESOFINDIA.COM
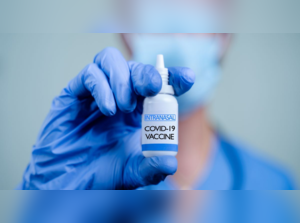
TIMESOFINDIA.COMThis will be India's first nasal vaccine for Covid-19, he added.
Mandaviya took to Twitter and wrote, "Big Boost to India's Fight Against COVID-19! Bharat Biotech's ChAd36-SARS-CoV-S COVID-19 (Chimpanzee Adenovirus Vectored) recombinant nasal vaccine approved by @CDSCO_INDIA_INF for primary immunization against COVID-19 in 18+ age group for restricted use in emergency situation."
The health minister said that the step will further strengthen India's collective fight against the pandemic.
"India has harnessed its science, R&D, and human resources in the fight against COVID-19 under PM @NarendraModi Ji's leadership. With the science-driven approach & Sabka Prayas, we will defeat COVID-19," he said.
(Catch all the Business News, Breaking News, Budget 2024 Events and Latest News Updates on The Economic Times.)
Subscribe to The Economic Times Prime and read the ET ePaper online.
Read More News on
(Catch all the Business News, Breaking News, Budget 2024 Events and Latest News Updates on The Economic Times.)
Subscribe to The Economic Times Prime and read the ET ePaper online.









 Get Unlimited Access to The Economic Times
Get Unlimited Access to The Economic Times
