DIABETES DRUG

Can Zaynich do for Wockhardt what Ozempic did for Novo Nordisk?
Wockhardt's Zaynich antibiotic surged stock by 75%. CEO Habil Khorakiwala cited its uniqueness; phase three trials end this fiscal year. Compared to Novo Nordisk's Ozempic, Zaynich’s $25 billion potential counters global antibiotic resistance.

Beware of weight loss medications; they can make you go blind
A recent study conducted by researchers at Massachusetts Eye and Ear hospital has found a significant link between popular weight loss drugs containing semaglutide and an increased risk of Non-Arteritic Anterior Ischemic Optic Neuropathy (NAION), a rare condition causing sudden vision loss in one eye. The study, published in JAMA Ophthalmology, revealed that obese patients using these medications were over seven times more likely to develop NAION, while diabetic patients faced more than four times the risk.

Waiting for a wonder drug? The weight just got longer
Eli Lilly awaits India's approval to sell Tirzepatide, marketed as Mounjaro and Zepbound in the US. CEO David Ricks plans to launch the drug in India in 2025, pending supply. The SEC recommends approval for pre-filled pens and vials, contingent on a phase-IV trial. The Drug Controller General of India will finalize the decision.

Weekly-once insulin jab may soon come to India
A new weekly insulin dosage by Novo Nordisk, Insulin Icodec, is on the brink of approval in India, offering potential relief for those who require daily insulin injections. The Subject Expert Committee has recommended permitting the import and sale of this innovative product, expected to revolutionise the insulin market. While further studies are required, doctors anticipate improved patient compliance with this new treatment option.
India to make drugs for diabetes, obesity under PLI by 2026, says report
India plans to incentivize local manufacturing of GLP-1 drugs, crucial for treating diabetes and obesity, starting in 2026, as per a government official speaking to Reuters. These drugs, originally approved for diabetes treatment, are also used for obesity due to their ability to slow digestion and reduce appetite. Novo Nordisk's patent on semaglutide, a key ingredient in popular drugs like Wegovy and Ozempic, is set to expire in India by 2026. Indian companies are already preparing to manufacture these drugs under the government's production-linked incentive (PLI) scheme.

Biocon seeks partner to test generic Wegovy, Ozempic in China
India's Biocon is seeking a Chinese partner to conduct clinical trials on generic versions of Novo Nordisk's popular diabetes drug Ozempic and weight loss treatment Wegovy. Biocon aims to eventually launch these generics globally, with plans to find a partner for the trials on over 500 patients in China, citing the substantial investment required. The move comes ahead of the patent protection expiry on key ingredients in the drugs in 2026.
- Go To Page 1

Ozempic frenzy lures rich Indians to brave the gray market
People are going to great lengths to obtain popular weight-loss drugs. They are stashing injectables in their carry-on luggage, buying counterfeit formulas online, and importing boxes from Europe. The treatments have sparked extensive media coverage, fueling a high demand. According to Goldman Sachs Research, the anti-obesity medication market could hit $100 billion by 2030. In contrast, Indians have largely been observers in this global frenzy over the new weight-loss solutions.

Govt allows global tenders to access 120 key, patented drugs
In what could ease access to some patented medicines, the government has allowed global tenders to be floated for procurement of 120 drugs, including anti-diabetic medication Semaglutide, after the health ministry raised concerns over lack of domestic options.

Ozempic: Bengaluru is the secret ingredient behind the wonder weight loss drugs' success
Novo Nordisk's India team played a pivotal role in the success of Ozempic, a semaglutide-based drug for diabetes and weight management. The Bengaluru-based Global Business Services unit collaborated on clinical trials and biostatistics. Despite global demand, Ozempic's availability in India remains uncertain due to supply challenges, with significant hiring planned to bolster Novo Nordisk's workforce in India.

Indoco Remedies gets USFDA nod to market generic diabetes drug
Indoco Remedies on Thursday said it has received approval from the US health regulator to market a generic diabetes medication. The company has received tentative approval from the US Food and Drug Administration (USFDA) for abbreviated new drug application (ANDA) for Canagliflozin and Metformin Hydrochloride tablets in various strengths, Indoco Remedies said in a regulatory filing.

Anti-diabetic drugs lead growth in pharmaceuticals market
The pharmaceuticals market in India is witnessing significant growth, with anti-diabetic drugs leading the way with over ₹155 crore in value growth among new brands launched in the past year. The respiratory segment and vitamin/mineral/nutrient segment followed closely behind. The anti-diabetes market has grown rapidly in the last five years, driven by factors like the increasing number of patients and the launch of new drug combinations. The pharmaceutical market rebounded in April 2024, with chronic segments showing faster growth compared to acute segments.

Don’t pop that pill daily: Doctors red-flag proton pump inhibitors used to treat acid reflux, heartburns
The reason for the popularity of PPIs among doctors and patients is not tough to gauge. With unhealthy lifestyles and dietary habits, acidity is common among Indians. While many brands of PPIs are sold almost over-the-counter, Pan, Pantop, Omez and Rablet fly off the chemist’s shelves. Doctors warn that overuse of these drugs can have a slow and debilitating impact.
USFDA approves Biocon Biologics' biosimilar product to treat ophthalmology conditions
Biocon Biologics received US Food and Drug Administration (USFDA) approval for biosimilar Yesafili, a vascular endothelial growth factor inhibitor for various ophthalmology conditions, including age-related macular degeneration. Yesafili is similar to Eylea. CEO Shreehas Tambe highlighted the company's milestone in bringing interchangeable insulin and biosimilars to patients. The approval comes as 19.8 million Americans live with macular degeneration, with aflibercept sales reaching $5.89 billion in 2023.

Uphill Battle: Navigating the Complexities of Diabetes Management
Managing type 2 diabetes involves crucial lifestyle changes for blood sugar control, mental health support, weight management, nutrition therapy, stress management, physical activity, and glucose regulation.
New oral diabetes + obesity drug's India sales surge 100%
India experiences a surge in Rybelsus sales, impacting the anti-obesity market. Semaglutide dominates, reflecting India's obesity challenges and evolving disease patterns as it grows as a superpower.

Sanofi introduces diabetes drug Soliqua in India
Sanofi India has launched its diabetes drug Soliqua in India, a once-daily injectable combination drug containing insulin and GLP-1 receptor agonist lixisenatide. The medication is available at a therapy cost of Rs 1,850 per pen and is indicated for adults with obesity and type 2 diabetes to improve glycemic control.
Dr Reddy’s, Sun, Cipla and Biocon look to recreate Ozempic magic in India
Doctors say patients come with specific queries regarding weight-loss drugs, which have become both the first preference and the last resort for some. “I would rather go for these short cuts that the pharma industry offers than go under the knife for my appearance,” says Rajput. The drugs — Wegovy and Ozempic — which have the same ingredient, semaglutide, are all the rage ever since entrepreneur Elon Musk tweeted about it.
‘Obese people should opt for a structured programme of diet, exercise & medication’
"Nothing comes without a downside. These molecules do produce significant nausea and vomiting in some people — to the extent that they have to discontinue the medication. Some may develop diarrhoea, some may have severe constipation. Others may not like the way they lose weight—they may feel very shrunken or their face may seem very thinned out. That’s another sort of downside," says Dr Ambrish Mithal, chairman, endocrinology & diabetes, Max Healthcare, Delhi.

Delhi HC upholds injunction against Glenmark Pharma
Sun Pharma Laboratories, a wholly-owned subsidiary of Sun Pharmaceutical Industries, and Glenmark are contesting over their right to use drug names “Istamet” and “Indamet”, respectively, which Sun Pharma finds to be deceptively similar and confusing.

Biocon partners Biomm to commercialise diabetes drug in Brazil
Biocon has partnered with Brazil-based Biomm SA for the commercialization of its diabetes drug, Semaglutide (gOzempic). Biocon will develop, manufacture, and supply the drug, while Biomm will obtain regulatory approval and commercialization in Brazil. Biomm focuses on developing, manufacturing, and commercialising complex biotech and biosimilar drug products.

Delhi High Court seeks response from govt, NPPA on Glenmark's plea
Delhi High Court hears Glenmark Pharma's petition against NPPA's pricing order for anti-diabetic drugs, claiming arbitrary pricing and seeking exemption due to DCGI approval.

Weight-loss medicines: Biocon eyes a $100 billion jackpot with weighty pivot
Weight-loss drugs: Biocon Ltd. is shifting focus to anti-obesity therapies as patents for key medications expire, leading to a surge in generic supply. The Bengaluru-based company secured UK approval for the first generic version of liraglutide injectible, positioning itself as a leader in this evolving market.

Sanofi plans to launch 1 or 2 products in India every year
Sanofi prioritizes the Indian market, focusing on diabetes, consumer health, and innovation. With a strategic shift under Hrosz's leadership, they aim to launch new products and enhance market reach through partnerships.

Novo Nordisk to buy Cardior Pharma for up to $1 billion
Novo Nordisk A/S bought Cardior Pharmaceuticals for $1 billion to enhance cardiovascular treatments. Cardior's microRNA-targeting therapies aim to improve heart function, aligning with Novo's focus on diabetes and obesity history.

Eris Lifesciences acquires Biocon Biologics domestic branded formulation business for Rs 1,242 crore
As part of the deal over 430 employees associated with the business are expected to transition to Eris, ensuring continuity for both employees and patients.
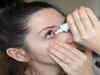
Roche launches top selling eye drug Vabysmo in India
Roche Pharma India launches Vabysmo, a medication for neovascular age-related macular degeneration and diabetic macular edema in India. The drug is cost-effective and collaborates with government and insurance companies for patient access. Vabysmo achieves global sales and offers durable treatment for retinal vision loss.

Weight-loss drug may be launched in India next year, says Eli Lilly CEO David Ricks
US drug maker Eli Lilly plans to launch its weight-loss drugs Zepbound and Mounjaro in India next year. The company is considering pricing based on supply and volume expansion. Eli Lilly CEO David Ricks suggests focusing on digital services in the Indian healthcare industry to encourage innovation and improve access to healthcare.

Glenmark launches antidiabetic drug in India
The drug is being marketed under the brand name Lirafit following the approval from the Drug Controller General of India (DCGI), the company said in a statement.
Load More
